Drug repurposing screens and synergistic drug-combinations for infectious diseases
- PMID: 28685814
- PMCID: PMC5758396
- DOI: 10.1111/bph.13895
Drug repurposing screens and synergistic drug-combinations for infectious diseases
Abstract
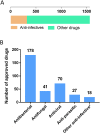
Infectious diseases account for nearly one fifth of the worldwide death toll every year. The continuous increase of drug-resistant pathogens is a big challenge for treatment of infectious diseases. In addition, outbreaks of infections and new pathogens are potential threats to public health. Lack of effective treatments for drug-resistant bacteria and recent outbreaks of Ebola and Zika viral infections have become a global public health concern. The number of newly approved antibiotics has decreased significantly in the last two decades compared with previous decades. In parallel with this, is an increase in the number of drug-resistant bacteria. For these threats and challenges to be countered, new strategies and technology platforms are critically needed. Drug repurposing has emerged as an alternative approach for rapid identification of effective therapeutics to treat the infectious diseases. For treatment of severe infections, synergistic drug combinations using approved drugs identified from drug repurposing screens is a useful option which may overcome the problem of weak activity of individual drugs. Collaborative efforts including government, academic researchers and private drug industry can facilitate the translational research to produce more effective new therapeutic agents such as narrow spectrum antibiotics against drug-resistant bacteria for these global challenges.
Linked articles: This article is part of a themed section on Inventing New Therapies Without Reinventing the Wheel: The Power of Drug Repurposing. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.2/issuetoc.
Published 2017. This article is a U.S. Government work and is in the public domain in the USA. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures

References
-
- Aldeyab MA, Monnet DL, Lopez‐Lozano JM, Hughes CM, Scott MG, Kearney MP et al. (2008). Modelling the impact of antibiotic use and infection control practices on the incidence of hospital‐acquired methicillin‐resistant Staphylococcus aureus: a time‐series analysis. J Antimicrob Chemother 62: 593–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

