Meta-analysis and critical review on the efficacy and safety of alpha-glucosidase inhibitors in Asian and non-Asian populations
- PMID: 28685995
- PMCID: PMC5835463
- DOI: 10.1111/jdi.12711
Meta-analysis and critical review on the efficacy and safety of alpha-glucosidase inhibitors in Asian and non-Asian populations
Abstract
Aims/introduction: To evaluate the efficacy and safety of alpha-glucosidase inhibitors (AGI) in Asian and non-Asian type 2 diabetes patients.
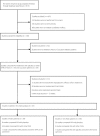
Materials and methods: Studies were identified through a literature search of MEDLINE, EMBASE and other databases until December 2016. All statistical analyses were carried out in Review Manager statistical software by computing the weighted mean difference or odds ratio and 95% confidence interval.
Results: A total of 67 studies were included. AGI vs placebo: compared with the placebo, AGI treatment led to a greater decrease in hemoglobin A1c (HbA1c), fasting plasma glucose and postprandial plasma glucose. No significant difference was observed in HbA1c change, fasting plasma glucose change, postprandial plasma glucose change or incidence of hypoglycemia between Asian and non-Asian patients. AGI vs active controls: in Asian patients, AGI treatment showed a lower reduction in HbA1c compared with dipeptidyl peptidase-4 inhibitors and sulfonylurea. In non-Asian patients, AGI treatment showed a lower reduction in HbA1c compared with thiazolidinedione. No significant difference was observed in HbA1c change and bodyweight change when comparing AGI with other oral hypoglycemic agents between Asian and non-Asian patients.
Conclusions: The effects of AGI treatment on glycemic control and bodyweight reduction were superior to the placebo without an increased incidence of hypoglycemia, but with an increased incidence of gastrointestinal discomforts. The hypoglycemic effects of AGI were comparable between Asian and non-Asian patients.
Keywords: Alpha-glucosidase inhibitors; Asian; Type 2 diabetes mellitus.
© 2017 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Figures
References
-
- Yang W, Lu J, Weng J, et al Prevalence of diabetes among men and women in China. N Engl J Med 2010; 362: 1090–1101. - PubMed
-
- Plus W. Pharmacology of Glucosidase Inhibitors, Vol. 119 Berlin Heidelberg: Springer, 1996; 611–632.
-
- Society CD . Chinese guideline for Type 2 diabetes prevention (2013). Chinese J Diabetes 2014; 22: 2–42.
-
- Chan JC, Chan KW, Ho LL, et al An Asian multicenter clinical trial to assess the efficacy and tolerability of acarbose compared with placebo in type 2 diabetic patients previously treated with diet, Asian Acarbose Study Group. Diabetes Care 1998; 21: 1058–1061. - PubMed
-
- Bachmann W, Petzinna D, Raptis SA, et al Long‐term improvement of metabolic control by acarbose in type 2 diabetes patients poorly controlled with maximum sulfonylurea therapy. Clin Drug Investig 2003; 23: 679–686. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical