Use of fresh-frozen plasma in 2012 at the Fondazione Ca' Granda Hospital of Milan: assessment of appropriateness using record linkage techniques applied to data routinely recorded in various hospital information systems
- PMID: 28686150
- PMCID: PMC5919837
- DOI: 10.2450/2017.0309-16
Use of fresh-frozen plasma in 2012 at the Fondazione Ca' Granda Hospital of Milan: assessment of appropriateness using record linkage techniques applied to data routinely recorded in various hospital information systems
Abstract
Background: The Quality Unit of a research and teaching hospital in Milan assessed the increased clinical use of fresh-frozen plasma in patients treated during 2012 in order to evaluate the appropriateness of this use.
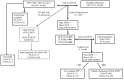
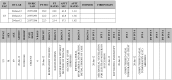
Materials and methods: For each patient in the study, a pathology profile was generated by means of record linkage techniques involving data collected through different information systems. Patients' information was combined using the patient identifier key generating pathology profiles exported to an Excel file. The profiles were reviewed by two haematologists who identified 101 potentially inappropriate treatments for which the medical records had to be reviewed manually.
Results: In 2012, 490 patients were transfused and for 473 cases the automatic record linkage provided a complete profile. The information relating to the remaining patients did not match, mainly because the patients underwent outpatient procedures for which clinical information is not automatically recorded. In the overall audit only 13 treatments were judged inappropriate.
Discussion: Our study supports the view that record linkage techniques applied to data routinely recorded in different hospital information systems could be potentially extended to support clinical audits, enabling the generation of automated patient profiles that can be easily evaluated, relegating manual checks on medical records to doubtful cases only. Moreover, the method applied in this study allows the analysis of a full set of cases instead of sample surveys, increasing the robustness of the audit results.
Conflict of interest statement
The Authors declare no conflicts of interest.
Figures
Similar articles
-
Three-year analysis of repeated laboratory tests for the markers total cholesterol, ferritin, vitamin D, vitamin B12 , and folate, in a large research and teaching hospital in Italy.J Eval Clin Pract. 2017 Jun;23(3):654-661. doi: 10.1111/jep.12696. Epub 2017 Jan 12. J Eval Clin Pract. 2017. PMID: 28078809
-
Appropriateness of transfusions of red cells, platelets and fresh frozen plasma. An audit in a tertiary care teaching hospital.Med J Aust. 1995 Jun 5;162(11):572-3, 576-7. Med J Aust. 1995. PMID: 7791642
-
Audit of the clinical use of fresh-frozen plasma in Umbria: study design and results of the pilot phase.Blood Transfus. 2008 Oct;6(4):211-9. doi: 10.2450/2008.0042-07. Blood Transfus. 2008. PMID: 19112736 Free PMC article.
-
[Clinical use of virally inactived plasma. The experience of Blood Transfusion Unit in Mantova, Italy].Recenti Prog Med. 2013 Mar;104(3):106-11. doi: 10.1701/1255.13858. Recenti Prog Med. 2013. PMID: 23548954 Review. Italian.
-
Appropriateness of fresh-frozen plasma usage in hospital settings: a meta-analysis of the impact of organizational interventions.Transfusion. 2010 Jan;50(1):139-44. doi: 10.1111/j.1537-2995.2009.02371.x. Epub 2009 Aug 31. Transfusion. 2010. PMID: 19719469
References
-
- EDQM - European Directorate for the Quality of Medicines & HealthCare. Guide to the Preparation, Use and Quality Assurance of Blood Components. 19th ed. 2016. [Accessed on: 30/10/2016]. Available at: https://www.edqm.eu/sites/default/files/list_of_contents_19th_ed-blood-q....
-
- Calizzani G, Lanzoni M, Candura F, editors. [Demand for the main blood medicinal products in Italy. Years 2007–2011]. Roma: Istituto Superiore di Sanità; 2012. (Rapporti ISTISAN 12/53) [In Italian.]
-
- Candura F, Lanzoni M, Calizzani G, et al. [Demand for the main blood medicinal products in Italy. Years 2011–2014]. Roma: Istituto Superiore di Sanità; 2016. (Rapporti ISTISAN 7/16) [In Italian.]
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
