Influence of red blood cell-derived microparticles upon vasoregulation
- PMID: 28686154
- PMCID: PMC5649961
- DOI: 10.2450/2017.0353-16
Influence of red blood cell-derived microparticles upon vasoregulation
Abstract
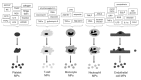
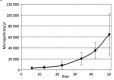
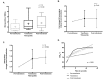
Here we review recent data and the evolving understanding of the role of red blood cell-derived microparticles (RMPs) in normal physiology and in disease progression. Microparticles (MPs) are small membrane vesicles derived from various parent cell types. MPs are produced in response to a variety of stimuli through several cytoskeletal and membrane phospholipid changes. MPs have been investigated as potential biomarkers for multiple disease processes and are thought to have biological effects, most notably in: promotion of coagulation, production and handling of reactive oxygen species, immune modulation, angiogenesis, and in apoptosis. Specifically, RMPs are produced normally during RBC maturation and their production is accelerated during processing and storage for transfusion. Several factors during RBC storage are known to trigger RMP production, including: increased intracellular calcium, increased potassium leakage, and energy failure with ATP depletion. Of note, RMP composition differs from that of intact RBCs, and the nature and composition of RMP components are affected by both storage duration and the character of storage solutions. Recognised RMP bioactivities include: promotion of coagulation, immune modulation, and promotion of endothelial adhesion, as well as influence upon vasoregulation via nitric oxide (NO) scavenging. Of particular relevance, RMPs are more avid NO scavengers than intact RBCs and this feature has been proposed as a mechanism for the impaired oxygen delivery homeostasis that has been observed following transfusion. Preliminary human studies demonstrate that circulating RMP abundance increases with RBC transfusion and is associated with altered plasma vasoactivity and abnormal vasoregulation. In summary, RMPs are submicron particles released from stored RBCs, with demonstrated vasoactive properties that appear to disturb oxygen delivery homeostasis. The clinical impact of RMPs in transfusion recipients is an area of continued investigation.
Conflict of interest statement
The Authors declare no conflicts of interest.
Figures





References
-
- Boulanger CM, Dignat-George F. Microparticles: an introduction. Arterioscler Thromb Vasc Biol. 2011;31:2–3. - PubMed
-
- Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31:15–26. - PubMed
-
- Burger D, Schock S, Thompson CS, et al. Microparticles: biomarkers and beyond. Clin Sci. 2013;124:423–41. - PubMed
-
- Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
