Multiple Sclerosis: Immunopathology and Treatment Update
- PMID: 28686222
- PMCID: PMC5532591
- DOI: 10.3390/brainsci7070078
Multiple Sclerosis: Immunopathology and Treatment Update
Abstract
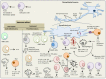
The treatment of multiple sclerosis (MS) has changed over the last 20 years. All immunotherapeutic drugs target relapsing remitting MS (RRMS) and it still remains a medical challenge in MS to develop a treatment for progressive forms. The most common injectable disease-modifying therapies in RRMS include β-interferons 1a or 1b and glatiramer acetate. However, one of the major challenges of injectable disease-modifying therapies has been poor treatment adherence with approximately 50% of patients discontinuing the therapy within the first year. Herein, we go back to the basics to understand the immunopathophysiology of MS to gain insights in the development of new improved drug treatments. We present current disease-modifying therapies (interferons, glatiramer acetate, dimethyl fumarate, teriflunomide, fingolimod, mitoxantrone), humanized monoclonal antibodies (natalizumab, ofatumumb, ocrelizumab, alentuzumab, daclizumab) and emerging immune modulating approaches (stem cells, DNA vaccines, nanoparticles, altered peptide ligands) for the treatment of MS.
Keywords: drug delivery; immunotherapy; multiple sclerosis; vaccine.
Conflict of interest statement
V.A. is supported by Vianex S.A. Greece in developing immunotherapeutics against MS; N.D. is supported under VU-Vianex contract 2 (specific task agreement MS immunotherapeutics) in developing immunotherapeutics against MS; J.M. is head of the scientific advisory board of ELDrug a spin off company of Vianex S.A.; M.-E.A. works for Vianex S.A. Greece; T.T. has an association with Vianex S.A. Greece in relation to supporting his research; M.K. is an employee of Novartis (Hellas) Greece; M.d.C. declares no conflicts of interest. The review represents a detailed literature search in the areas of drugs and treatments against MS with no bias towards immunotherapeutics developed by Vianex S.A.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources