Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage
- PMID: 28686597
- PMCID: PMC5521874
- DOI: 10.1371/journal.pgen.1006897
Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage
Abstract
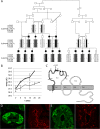
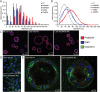
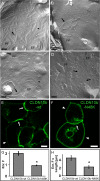
Claudins constitute the major component of tight junctions and regulate paracellular permeability of epithelia. Claudin-10 occurs in two major isoforms that form paracellular channels with ion selectivity. We report on two families segregating an autosomal recessive disorder characterized by generalized anhidrosis, severe heat intolerance and mild kidney failure. All affected individuals carry a rare homozygous missense mutation c.144C>G, p.(N48K) specific for the claudin-10b isoform. Immunostaining of sweat glands from patients suggested that the disease is associated with reduced levels of claudin-10b in the plasma membranes and in canaliculi of the secretory portion. Expression of claudin-10b N48K in a 3D cell model of sweat secretion indicated perturbed paracellular Na+ transport. Analysis of paracellular permeability revealed that claudin-10b N48K maintained cation over anion selectivity but with a reduced general ion conductance. Furthermore, freeze fracture electron microscopy showed that claudin-10b N48K was associated with impaired tight junction strand formation and altered cis-oligomer formation. These data suggest that claudin-10b N48K causes anhidrosis and our findings are consistent with a combined effect from perturbed TJ function and increased degradation of claudin-10b N48K in the sweat glands. Furthermore, affected individuals present with Mg2+ retention, secondary hyperparathyroidism and mild kidney failure that suggest a disturbed reabsorption of cations in the kidneys. These renal-derived features recapitulate several phenotypic aspects detected in mice with kidney specific loss of both claudin-10 isoforms. Our study adds to the spectrum of phenotypes caused by tight junction proteins and demonstrates a pivotal role for claudin-10b in maintaining paracellular Na+ permeability for sweat production and kidney function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Liang GH, Weber CR. Molecular aspects of tight junction barrier function. Current opinion in pharmacology. 2014;19:84–9. Epub 2014/08/17. doi: 10.1016/j.coph.2014.07.017 ; PubMed Central PMCID: PMCPmc4330960. - DOI - PMC - PubMed
-
- Gunzel D, Fromm M. Claudins and other tight junction proteins. Comprehensive Physiology. 2012;2(3):1819–52. Epub 2013/06/01. doi: 10.1002/cphy.c110045 . - DOI - PubMed
-
- Krug SM, Gunzel D, Conrad MP, Lee IF, Amasheh S, Fromm M, et al. Charge-selective claudin channels. Ann N Y Acad Sci. 2012;1257:20–8. Epub 2012/06/08. doi: 10.1111/j.1749-6632.2012.06555.x . - DOI - PubMed
-
- Gunzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93(2):525–69. Epub 2013/04/17. doi: 10.1152/physrev.00019.2012 ; PubMed Central PMCID: PMCPMC3768107. - DOI - PMC - PubMed
-
- Piontek J, Winkler L, Wolburg H, Muller SL, Zuleger N, Piehl C, et al. Formation of tight junction: determinants of homophilic interaction between classic claudins. Faseb J. 2008;22(1):146–58. Epub 2007/09/01. doi: 10.1096/fj.07-8319com . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

