Genotyping assay for differentiation of wild-type and vaccine viruses in subjects immunized with live attenuated influenza vaccine
- PMID: 28686625
- PMCID: PMC5501548
- DOI: 10.1371/journal.pone.0180497
Genotyping assay for differentiation of wild-type and vaccine viruses in subjects immunized with live attenuated influenza vaccine
Abstract
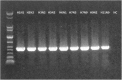
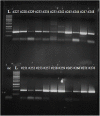
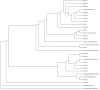
Live attenuated influenza vaccines (LAIVs) are considered as safe and effective tool to control influenza in different age groups, especially in young children. An important part of the LAIV safety evaluation is the detection of vaccine virus replication in the nasopharynx of the vaccinees, with special attention to a potential virus transmission to the unvaccinated close contacts. Conducting LAIV clinical trials in some geographical regions with year-round circulation of influenza viruses warrants the development of robust and reliable tools for differentiating vaccine viruses from wild-type influenza viruses in nasal pharyngeal wash (NPW) specimens of vaccinated subjects. Here we report the development of genotyping assay for the detection of wild-type and vaccine-type influenza virus genes in NPW specimens of young children immunized with Russian-backbone seasonal trivalent LAIV using Sanger sequencing from newly designed universal primers. The new primer set allowed amplification and sequencing of short fragments of viral genes in NPW specimens and appeared to be more sensitive than conventional real-time RT-PCR protocols routinely used for the detection and typing/subtyping of influenza virus in humans. Furthermore, the new assay is capable of defining the origin of wild-type influenza virus through BLAST search with the generated sequences of viral genes fragments.
Conflict of interest statement
Figures

References
-
- Nair H, Brooks WA, Katz M, Roca A, Berkley JA, et al. (2011) Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 378: 1917–1930. doi: 10.1016/S0140-6736(11)61051-9 - DOI - PubMed
-
- Ortiz JR, Goswami D, Lewis KD, Sharmeen AT, Ahmed M, et al. (2015) Safety of Russian-backbone seasonal trivalent, live-attenuated influenza vaccine in a phase II randomized placebo-controlled clinical trial among children in urban Bangladesh. Vaccine. - PubMed
-
- Block SL, Yogev R, Hayden FG, Ambrose CS, Zeng W, et al. (2008) Shedding and immunogenicity of live attenuated influenza vaccine virus in subjects 5–49 years of age. Vaccine 26: 4940–4946. doi: 10.1016/j.vaccine.2008.07.013 - DOI - PubMed
-
- Vesikari T, Karvonen A, Korhonen T, Edelman K, Vainionpaa R, et al. (2006) A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J 25: 590–595. doi: 10.1097/01.inf.0000220229.51531.47 - DOI - PubMed
-
- Mallory RM, Yi T, Ambrose CS (2011) Shedding of Ann Arbor strain live attenuated influenza vaccine virus in children 6–59 months of age. Vaccine 29: 4322–4327. doi: 10.1016/j.vaccine.2011.04.022 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

