Phase I randomized clinical trial of N-acetylcysteine in combination with an adjuvant probenecid for treatment of severe traumatic brain injury in children
- PMID: 28686657
- PMCID: PMC5501440
- DOI: 10.1371/journal.pone.0180280
Phase I randomized clinical trial of N-acetylcysteine in combination with an adjuvant probenecid for treatment of severe traumatic brain injury in children
Abstract
Background: There are no therapies shown to improve outcome after severe traumatic brain injury (TBI) in humans, a leading cause of morbidity and mortality. We sought to verify brain exposure of the systemically administered antioxidant N-acetylcysteine (NAC) and the synergistic adjuvant probenecid, and identify adverse effects of this drug combination after severe TBI in children.
Methods: IRB-approved, randomized, double-blind, placebo controlled Phase I study in children 2 to 18 years-of-age admitted to a Pediatric Intensive Care Unit after severe TBI (Glasgow Coma Scale [GCS] score ≤8) requiring an externalized ventricular drain for measurement of intracranial pressure (ICP). Patients were recruited from November 2011-August 2013. Fourteen patients (n = 7/group) were randomly assigned after obtaining informed consent to receive probenecid (25 mg/kg load, then 10 mg/kg/dose q6h×11 doses) and NAC (140 mg/kg load, then 70 mg/kg/dose q4h×17 doses), or placebos via naso/orogastric tube. Serum and CSF samples were drawn pre-bolus and 1-96 h after randomization and drug concentrations were measured via UPLC-MS/MS. Glasgow Outcome Scale (GOS) score was assessed at 3 months.
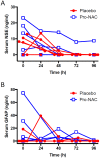
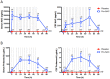
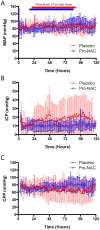
Results: There were no adverse events attributable to drug treatment. One patient in the placebo group was withdrawn due to adverse effects. In the treatment group, NAC concentrations ranged from 16,977.3±2,212.3 to 16,786.1±3,285.3 in serum and from 269.3±113.0 to 467.9±262.7 ng/mL in CSF, at 24 to 72 h post-bolus, respectively; and probenecid concentrations ranged from 75.4.3±10.0 to 52.9±25.8 in serum and 5.4±1.0 to 4.6±2.1 μg/mL in CSF, at 24 to 72 h post-bolus, respectively (mean±SEM). Temperature, mean arterial pressure, ICP, use of ICP-directed therapies, surveillance serum brain injury biomarkers, and GOS at 3 months were not different between groups.
Conclusions: Treatment resulted in detectable concentrations of NAC and probenecid in CSF and was not associated with undesirable effects after TBI in children.
Trial registration: ClinicalTrials.gov NCT01322009.
Conflict of interest statement
Figures
References
-
- CDC. 2015. http://www.cdc.gov/nchs/fastats/child-health.htm.
-
- Tolias CM, Bullock MR. Critical appraisal of neuroprotection trials in head injury: what have we learned? NeuroRx: The journal of the American Society for Experimental NeuroTherapeutics. 2004;1(1):71–9. Epub 2005/02/18. doi: 10.1602/neurorx.1.1.71 ; - DOI - PMC - PubMed
-
- Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014;371(26):2457–66. Epub 2014/12/11. doi: 10.1056/NEJMoa1404304 . - DOI - PMC - PubMed
-
- Du L, Empey PE, Ji J, Chao H, Kochanek PM, Bayir H, et al. Probenecid and N-Acetylcysteine Prevent Loss of Intracellular Glutathione and Inhibit Neuronal Death after Mechanical Stretch Injury In Vitro. J Neurotrauma. 2016;33(20):1913–7. doi: 10.1089/neu.2015.4342 ; - DOI - PMC - PubMed
-
- Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84(5):1173–83. . - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

