Drosophila lines with mutant and wild type human TDP-43 replacing the endogenous gene reveals phosphorylation and ubiquitination in mutant lines in the absence of viability or lifespan defects
- PMID: 28686708
- PMCID: PMC5501610
- DOI: 10.1371/journal.pone.0180828
Drosophila lines with mutant and wild type human TDP-43 replacing the endogenous gene reveals phosphorylation and ubiquitination in mutant lines in the absence of viability or lifespan defects
Abstract
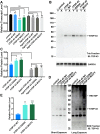
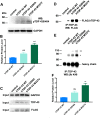
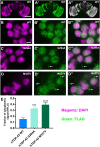
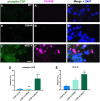
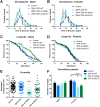
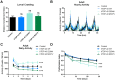
Mutations in TDP-43 are associated with proteinaceous inclusions in neurons and are believed to be causative in neurodegenerative diseases such as frontotemporal dementia or amyotrophic lateral sclerosis. Here we describe a Drosophila system where we have engineered the genome to replace the endogenous TDP-43 orthologue with wild type or mutant human TDP-43(hTDP-43). In contrast to other models, these flies express both mutant and wild type hTDP-43 at similar levels to those of the endogenous gene and importantly, no age-related TDP-43 accumulation observed among all the transgenic fly lines. Immunoprecipitation of TDP-43 showed that flies with hTDP-43 mutations had increased levels of ubiquitination and phosphorylation of the hTDP-43 protein. Furthermore, histologically, flies expressing hTDP-43 M337V showed global, robust neuronal staining for phospho-TDP. All three lines: wild type hTDP-43, -G294A and -M337V were homozygous viable, with no defects in development, life span or behaviors observed. The primary behavioral defect was that flies expressing either hTDP-43 G294A or M337V showed a faster decline with age in negative geotaxis. Together, these observations implied that neurons could handle these TDP-43 mutations by phosphorylation- and ubiquitin-dependent proteasome systems, even in a background without the wild type TDP-43. Our findings suggest that these two specific TDP-43 mutations are not inherently toxic, but may require additional environmental or genetic factors to affect longevity or survival.
Conflict of interest statement
Figures

References
-
- Heemels MT. Neurodegenerative diseases. Nature. 2016;539(7628):179 Epub 2016/11/11. doi: 10.1038/539179a . - DOI - PubMed
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochemical and biophysical research communications. 2006;351(3):602–11. doi: 10.1016/j.bbrc.2006.10.093 . - DOI - PubMed
-
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–3. doi: 10.1126/science.1134108 . - DOI - PubMed
-
- Banks GT, Kuta A, Isaacs AM, Fisher EM. TDP-43 is a culprit in human neurodegeneration, and not just an innocent bystander. Mammalian genome: official journal of the International Mammalian Genome Society. 2008;19(5):299–305. Epub 2008/07/02. doi: 10.1007/s00335-008-9117-x . - DOI - PMC - PubMed
-
- Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, et al. Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Archives of neurology. 2009;66(2):180–9. Epub 2009/02/11. doi: 10.1001/archneurol.2008.558 . - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

