A Pentanucleotide ATTTC Repeat Insertion in the Non-coding Region of DAB1, Mapping to SCA37, Causes Spinocerebellar Ataxia
- PMID: 28686858
- PMCID: PMC5501871
- DOI: 10.1016/j.ajhg.2017.06.007
A Pentanucleotide ATTTC Repeat Insertion in the Non-coding Region of DAB1, Mapping to SCA37, Causes Spinocerebellar Ataxia
Abstract
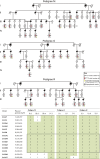
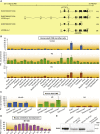
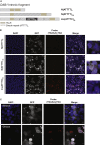
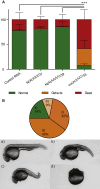
Advances in human genetics in recent years have largely been driven by next-generation sequencing (NGS); however, the discovery of disease-related gene mutations has been biased toward the exome because the large and very repetitive regions that characterize the non-coding genome remain difficult to reach by that technology. For autosomal-dominant spinocerebellar ataxias (SCAs), 28 genes have been identified, but only five SCAs originate from non-coding mutations. Over half of SCA-affected families, however, remain without a genetic diagnosis. We used genome-wide linkage analysis, NGS, and repeat analysis to identify an (ATTTC)n insertion in a polymorphic ATTTT repeat in DAB1 in chromosomal region 1p32.2 as the cause of autosomal-dominant SCA; this region has been previously linked to SCA37. The non-pathogenic and pathogenic alleles have the configurations [(ATTTT)7-400] and [(ATTTT)60-79(ATTTC)31-75(ATTTT)58-90], respectively. (ATTTC)n insertions are present on a distinct haplotype and show an inverse correlation between size and age of onset. In the DAB1-oriented strand, (ATTTC)n is located in 5' UTR introns of cerebellar-specific transcripts arising mostly during human fetal brain development from the usage of alternative promoters, but it is maintained in the adult cerebellum. Overexpression of the transfected (ATTTC)58 insertion, but not (ATTTT)n, leads to abnormal nuclear RNA accumulation. Zebrafish embryos injected with RNA of the (AUUUC)58 insertion, but not (AUUUU)n, showed lethal developmental malformations. Together, these results establish an unstable repeat insertion in DAB1 as a cause of cerebellar degeneration; on the basis of the genetic and phenotypic evidence, we propose this mutation as the molecular basis for SCA37.
Keywords: DAB1 reelin adaptor protein; RNA-mediated toxicity; SCA37; large Alu pentanucleotide repeat; neurodegeneration; neurodegenerative disease; neurodevelopmental gene; repeat expansion; repeat instability; unstable repeat insertion.
Copyright © 2017 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Sequeiros J., Martins S., Silveira I. Epidemiology and population genetics of degenerative ataxias. Handb. Clin. Neurol. 2012;103:227–251. - PubMed
-
- Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–894. - PubMed
-
- Coutinho P., Ruano L., Loureiro J.L., Cruz V.T., Barros J., Tuna A., Barbot C., Guimarães J., Alonso I., Silveira I. Hereditary ataxia and spastic paraplegia in Portugal: a population-based prevalence study. JAMA Neurol. 2013;70:746–755. - PubMed
-
- Silveira I., Miranda C., Guimarães L., Moreira M.C., Alonso I., Mendonça P., Ferro A., Pinto-Basto J., Coelho J., Ferreirinha F. Trinucleotide repeats in 202 families with ataxia: a small expanded (CAG)n allele at the SCA17 locus. Arch. Neurol. 2002;59:623–629. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

