Gemcitabine enhances the transport of nanovector-albumin-bound paclitaxel in gemcitabine-resistant pancreatic ductal adenocarcinoma
- PMID: 28687352
- PMCID: PMC5560598
- DOI: 10.1016/j.canlet.2017.06.026
Gemcitabine enhances the transport of nanovector-albumin-bound paclitaxel in gemcitabine-resistant pancreatic ductal adenocarcinoma
Abstract
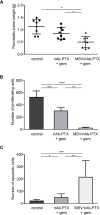
The mechanism for improved therapeutic efficacy of the combination therapy with nanoparticle albumin-bound paclitaxel (nAb-PTX) and gemcitabine (gem) for pancreatic ductal adenocarcinoma (PDAC) has been ascribed to enhanced gem transport by nAb-PTX. Here, we used an orthotopic mouse model of gem-resistant human PDAC in which increasing gem transport would not improve the efficacy, thus revealing the importance of nAb-PTX transport. We aimed to evaluate therapeutic outcomes and transport of nAb-PTX to PDAC as a result of (1) encapsulating nAb-PTX in multistage nanovectors (MSV); (2) effect of gem on caveolin-1 expression. Treatment with MSV/nAb-PTX + gem was highly efficient in prolonging animal survival in comparison to other therapeutic regimens. MSV/nAb-PTX + gem also caused a substantial increase in tumor PTX accumulation, significantly reduced tumor growth and tumor cell proliferation, and increased apoptosis. Moreover, gem enhanced caveolin-1 expression in vitro and in vivo, thereby improving transport of nAb-PTX to PDAC. This data was confirmed by analysis of PDACs from patients who received gem-based neo-adjuvant chemotherapy. In conclusion, we found that nAb-PTX treatment of gem-resistant PDAC can be enhanced by (1) gem through up-regulation of caveolin-1 and (2) MSV through increasing accumulation of nAb-PTX in the tumor.
Keywords: Drug resistance; Gemcitabine; Multistage nanovectors; Pancreatic cancer; Transport; nAb-paclitaxel.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
M. Ferrari is the founding scientist and a member of the Board of Directors of NanoMedical Systems, and a member of Board of Directors of Arrowhead Research Corporation, and hereby discloses potential financial interests in the companies. No potential conflicts of interest were disclosed by the other authors.
Figures





Similar articles
-
Superior therapeutic efficacy of nab-paclitaxel over cremophor-based paclitaxel in locally advanced and metastatic models of human pancreatic cancer.Br J Cancer. 2016 Aug 9;115(4):442-53. doi: 10.1038/bjc.2016.215. Epub 2016 Jul 21. Br J Cancer. 2016. PMID: 27441498 Free PMC article.
-
Altered Gemcitabine and Nab-paclitaxel Scheduling Improves Therapeutic Efficacy Compared with Standard Concurrent Treatment in Preclinical Models of Pancreatic Cancer.Clin Cancer Res. 2021 Jan 15;27(2):554-565. doi: 10.1158/1078-0432.CCR-20-1422. Epub 2020 Oct 21. Clin Cancer Res. 2021. PMID: 33087331 Free PMC article.
-
OSI-027 inhibits pancreatic ductal adenocarcinoma cell proliferation and enhances the therapeutic effect of gemcitabine both in vitro and in vivo.Oncotarget. 2015 Sep 22;6(28):26230-41. doi: 10.18632/oncotarget.4579. Oncotarget. 2015. PMID: 26213847 Free PMC article.
-
Nano albumin bound-paclitaxel in pancreatic cancer: Current evidences and future directions.World J Gastroenterol. 2017 Aug 28;23(32):5875-5886. doi: 10.3748/wjg.v23.i32.5875. World J Gastroenterol. 2017. PMID: 28932079 Free PMC article. Review.
-
Do anti-stroma therapies improve extrinsic resistance to increase the efficacy of gemcitabine in pancreatic cancer?Cell Mol Life Sci. 2018 Mar;75(6):1001-1012. doi: 10.1007/s00018-017-2678-7. Epub 2017 Oct 9. Cell Mol Life Sci. 2018. PMID: 28993833 Free PMC article. Review.
Cited by
-
Role of Caveolae family-related proteins in the development of breast cancer.Front Mol Biosci. 2023 Sep 27;10:1242426. doi: 10.3389/fmolb.2023.1242426. eCollection 2023. Front Mol Biosci. 2023. PMID: 37828916 Free PMC article. Review.
-
Estrogen receptor α inhibits Caveolin 1 translation by promoting m6A-dependent miR199a-5p maturation to confer nab-paclitaxel resistance.Am J Cancer Res. 2023 Dec 15;13(12):6210-6225. eCollection 2023. Am J Cancer Res. 2023. PMID: 38187046 Free PMC article.
-
Modeling of Nanotherapy Response as a Function of the Tumor Microenvironment: Focus on Liver Metastasis.Front Bioeng Biotechnol. 2020 Aug 19;8:1011. doi: 10.3389/fbioe.2020.01011. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32974325 Free PMC article. Review.
-
Biodistribution and therapeutic efficacy of a gold nanoparticle-based targeted drug delivery system against pancreatic cancer.Cancer Lett. 2024 May 1;589:216810. doi: 10.1016/j.canlet.2024.216810. Epub 2024 Mar 15. Cancer Lett. 2024. PMID: 38494151 Free PMC article.
-
Optimize the combination regimen of Trastuzumab and Nab-paclitaxel in HER2-positive tumors via modulating Caveolin-1 expression by lovastatin.Asian J Pharm Sci. 2022 Aug;17(5):697-712. doi: 10.1016/j.ajps.2022.06.002. Epub 2022 Jul 28. Asian J Pharm Sci. 2022. PMID: 36382307 Free PMC article.
References
-
- Malvezzi M, Bertuccio P, Rosso T, Rota M, Levi F, La Vecchia C, et al. European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women? Ann Oncol. 2015;26(4):779–86. - PubMed
-
- American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015.
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. - PubMed
-
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

