Endothelial NF- κ B Blockade Abrogates ANCA-Induced GN
- PMID: 28687535
- PMCID: PMC5661273
- DOI: 10.1681/ASN.2016060690
Endothelial NF- κ B Blockade Abrogates ANCA-Induced GN
Abstract
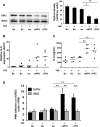
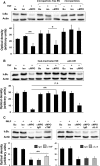
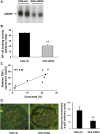
ANCA-associated vasculitis (AAV) is a highly inflammatory condition in which ANCA-activated neutrophils interact with the endothelium, resulting in necrotizing vasculitis. We tested the hypothesis that endothelial NF-κB mediates necrotizing crescentic GN (NCGN) and provides a specific treatment target. Reanalysis of kidneys from previously examined murine NCGN disease models revealed NF-κB activation in affected kidneys, mostly as a p50/p65 heterodimer, and increased renal expression of NF-κB-dependent tumor necrosis factor α (TNF-α). NF-κB activation positively correlated with crescent formation, and nuclear phospho-p65 staining showed NF-κB activation within CD31-expressing endothelial cells (ECs) in affected glomeruli. Therefore, we studied the effect of ANCA on NF-κB activation in neutrophil/EC cocultures in vitro ANCA did not activate NF-κB in primed human neutrophils, but ANCA-stimulated primed neutrophils activated NF-κB in ECs, at least in part via TNF-α release. This effect increased endothelial gene transcription and protein production of NF-κB-regulated interleukin-8. Moreover, upregulation of endothelial NF-κB promoted neutrophil adhesion to EC monolayers, an effect that was inhibited by a specific IKKβ inhibitor. In a murine NCGN model, prophylactic application of E-selectin-targeted immunoliposomes packed with p65 siRNA to downregulate endothelial NF-κB significantly reduced urine abnormalities, renal myeloid cell influx, and NCGN. Increased glomerular endothelial phospho-p65 staining in patients with AAV indicated that NF-κB is activated in human NCGN also. We suggest that ANCA-stimulated neutrophils activate endothelial NF-κB, which contributes to NCGN and provides a potential therapeutic target in AAV.
Keywords: ANCA; endothelium; glomerulonephritis; transcription factors; vasculitis.
Copyright © 2017 by the American Society of Nephrology.
Figures





Similar articles
-
Impact of anti-glomerular basement membrane antibodies and glomerular neutrophil activation on glomerulonephritis in experimental myeloperoxidase-antineutrophil cytoplasmic antibody vasculitis.Nephrol Dial Transplant. 2016 Apr;31(4):574-85. doi: 10.1093/ndt/gfv384. Epub 2015 Nov 17. Nephrol Dial Transplant. 2016. PMID: 26582929
-
Neutrophil Gelatinase-Associated Lipocalin Protects from ANCA-Induced GN by Inhibiting TH17 Immunity.J Am Soc Nephrol. 2020 Jul;31(7):1569-1584. doi: 10.1681/ASN.2019090879. Epub 2020 Jun 2. J Am Soc Nephrol. 2020. PMID: 32487561 Free PMC article.
-
Therapeutic Myeloperoxidase Inhibition Attenuates Neutrophil Activation, ANCA-Mediated Endothelial Damage, and Crescentic GN.J Am Soc Nephrol. 2020 Feb;31(2):350-364. doi: 10.1681/ASN.2019060618. Epub 2019 Dec 26. J Am Soc Nephrol. 2020. PMID: 31879336 Free PMC article.
-
New advances in the pathogenesis of ANCA-associated vasculitides.Clin Exp Rheumatol. 2009 Jan-Feb;27(1 Suppl 52):S108-14. Clin Exp Rheumatol. 2009. PMID: 19646356 Review.
-
The rise of complement in ANCA-associated vasculitis: from marginal player to target of modern therapy.Clin Exp Immunol. 2020 Dec;202(3):403-406. doi: 10.1111/cei.13515. Epub 2020 Oct 5. Clin Exp Immunol. 2020. PMID: 32946609 Free PMC article. Review.
Cited by
-
Targeted Transfection Using PEGylated Cationic Liposomes Directed Towards P-Selectin Increases siRNA Delivery into Activated Endothelial Cells.Pharmaceutics. 2019 Jan 21;11(1):47. doi: 10.3390/pharmaceutics11010047. Pharmaceutics. 2019. PMID: 30669699 Free PMC article.
-
ATG5-mediated autophagy suppresses NF-κB signaling to limit epithelial inflammatory response to kidney injury.Cell Death Dis. 2019 Mar 15;10(4):253. doi: 10.1038/s41419-019-1483-7. Cell Death Dis. 2019. PMID: 30874544 Free PMC article.
-
The TLR4-MyD88-NF-κB pathway is involved in sIgA-mediated IgA nephropathy.J Nephrol. 2020 Dec;33(6):1251-1261. doi: 10.1007/s40620-020-00722-3. Epub 2020 May 9. J Nephrol. 2020. PMID: 32388684 Free PMC article.
-
Animal models for anti-neutrophil cytoplasmic antibody-associated vasculitis: Are current models good enough?Animal Model Exp Med. 2023 Oct;6(5):452-463. doi: 10.1002/ame2.12345. Epub 2023 Aug 23. Animal Model Exp Med. 2023. PMID: 37614099 Free PMC article. Review.
-
Toll-like receptor signaling pathway involved in pathogenesis of thromboangiitis obliterans through activating of NF-κB.Clinics (Sao Paulo). 2024 Apr 18;79:100357. doi: 10.1016/j.clinsp.2024.100357. eCollection 2024. Clinics (Sao Paulo). 2024. PMID: 38640750 Free PMC article.
References
-
- Kettritz R, Schreiber A, Luft FC, Haller H: Role of mitogen-activated protein kinases in activation of human neutrophils by antineutrophil cytoplasmic antibodies. J Am Soc Nephrol 12: 37–46, 2001 - PubMed
-
- Ben-Smith A, Dove SK, Martin A, Wakelam MJ, Savage CO: Antineutrophil cytoplasm autoantibodies from patients with systemic vasculitis activate neutrophils through distinct signaling cascades: Comparison with conventional Fcgamma receptor ligation. Blood 98: 1448–1455, 2001 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

