Regulator of G-protein signaling Gβ5-R7 is a crucial activator of muscarinic M3 receptor-stimulated insulin secretion
- PMID: 28687610
- PMCID: PMC5636707
- DOI: 10.1096/fj.201700197RR
Regulator of G-protein signaling Gβ5-R7 is a crucial activator of muscarinic M3 receptor-stimulated insulin secretion
Abstract
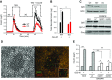
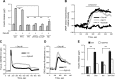
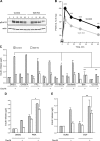
In pancreatic β cells, muscarinic cholinergic receptor M3 (M3R) stimulates glucose-induced secretion of insulin. Regulator of G-protein signaling (RGS) proteins are critical modulators of GPCR activity, yet their role in β cells remains largely unknown. R7 subfamily RGS proteins are stabilized by the G-protein subunit Gβ5, such that the knockout of the Gnb5 gene results in degradation of all R7 subunits. We found that Gnb5 knockout in mice or in the insulin-secreting MIN6 cell line almost completely eliminates insulinotropic activity of M3R. Moreover, overexpression of Gβ5-RGS7 strongly promotes M3R-stimulated insulin secretion. Examination of this noncanonical mechanism in Gnb5-/- MIN6 cells showed that cAMP, diacylglycerol, or Ca2+ levels were not significantly affected. There was no reduction in the amplitude of free Ca2+ responses in islets from the Gnb5-/- mice, but the frequency of Ca2+ oscillations induced by cholinergic agonist was lowered by more than 30%. Ablation of Gnb5 impaired M3R-stimulated phosphorylation of ERK1/2. Stimulation of the ERK pathway in Gnb5-/- cells by epidermal growth factor restored M3R-stimulated insulin release to near normal levels. Identification of the novel role of Gβ5-R7 in insulin secretion may lead to a new therapeutic approach for improving pancreatic β-cell function.-Wang, Q., Pronin, A. N., Levay, K., Almaca, J., Fornoni, A., Caicedo, A., Slepak, V. Z. Regulator of G-protein signaling Gβ5-R7 is a crucial activator of muscarinic M3 receptor-stimulated insulin secretion.
Keywords: CRISPR-Cas9; UBO-QIC; fluorescent biosensor; islet of Langerhans; pupil sphincter.
© FASEB.
Figures


References
-
- Moran B. M., Flatt P. R., McKillop A. M. (2016) G protein-coupled receptors: signalling and regulation by lipid agonists for improved glucose homoeostasis. Acta Diabetol. 53, 177–188 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

