Posttranscriptional Regulation of PARG mRNA by HuR Facilitates DNA Repair and Resistance to PARP Inhibitors
- PMID: 28687616
- PMCID: PMC5663502
- DOI: 10.1158/0008-5472.CAN-16-2704
Posttranscriptional Regulation of PARG mRNA by HuR Facilitates DNA Repair and Resistance to PARP Inhibitors
Abstract
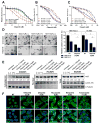
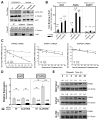
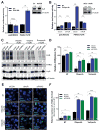
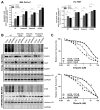
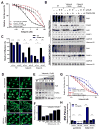
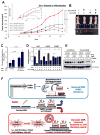
The majority of pancreatic ductal adenocarcinomas (PDAC) rely on the mRNA stability factor HuR (ELAV-L1) to drive cancer growth and progression. Here, we show that CRISPR-Cas9-mediated silencing of the HuR locus increases the relative sensitivity of PDAC cells to PARP inhibitors (PARPi). PDAC cells treated with PARPi stimulated translocation of HuR from the nucleus to the cytoplasm, specifically promoting stabilization of a new target, poly (ADP-ribose) glycohydrolase (PARG) mRNA, by binding a unique sequence embedded in its 3' untranslated region. HuR-dependent upregulation of PARG expression facilitated DNA repair via hydrolysis of polyADP-ribose on related repair proteins. Accordingly, strategies to inhibit HuR directly promoted DNA damage accumulation, inefficient PAR removal, and persistent PARP-1 residency on chromatin (PARP-1 trapping). Immunoprecipitation assays demonstrated that the PARP-1 protein binds and posttranslationally modifies HuR in PARPi-treated PDAC cells. In a mouse xenograft model of human PDAC, PARPi monotherapy combined with targeted silencing of HuR significantly reduced tumor growth compared with PARPi therapy alone. Our results highlight the HuR-PARG axis as an opportunity to enhance PARPi-based therapies. Cancer Res; 77(18); 5011-25. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures
Similar articles
-
The BET inhibitor JQ1 attenuates double-strand break repair and sensitizes models of pancreatic ductal adenocarcinoma to PARP inhibitors.EBioMedicine. 2019 Jun;44:419-430. doi: 10.1016/j.ebiom.2019.05.035. Epub 2019 May 22. EBioMedicine. 2019. PMID: 31126889 Free PMC article.
-
Human antigen R mediated post-transcriptional regulation of inhibitors of apoptosis proteins in pancreatic cancer.World J Gastroenterol. 2019 Jan 14;25(2):205-219. doi: 10.3748/wjg.v25.i2.205. World J Gastroenterol. 2019. PMID: 30670910 Free PMC article.
-
Poly (ADP) Ribose Glycohydrolase Can Be Effectively Targeted in Pancreatic Cancer.Cancer Res. 2019 Sep 1;79(17):4491-4502. doi: 10.1158/0008-5472.CAN-18-3645. Epub 2019 Jul 4. Cancer Res. 2019. PMID: 31273064 Free PMC article.
-
Targeting poly(ADP-ribose) glycohydrolase to draw apoptosis codes in cancer.Biochem Pharmacol. 2019 Sep;167:163-172. doi: 10.1016/j.bcp.2019.06.004. Epub 2019 Jun 6. Biochem Pharmacol. 2019. PMID: 31176615 Review.
-
PARP inhibitor resistance: the underlying mechanisms and clinical implications.Mol Cancer. 2020 Jun 20;19(1):107. doi: 10.1186/s12943-020-01227-0. Mol Cancer. 2020. PMID: 32563252 Free PMC article. Review.
Cited by
-
Roles of Embryonic Lethal Abnormal Vision-Like RNA Binding Proteins in Cancer and Beyond.Front Cell Dev Biol. 2022 Apr 6;10:847761. doi: 10.3389/fcell.2022.847761. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35465324 Free PMC article. Review.
-
The RNA-Binding Protein HuR Posttranscriptionally Regulates the Protumorigenic Activator YAP1 in Pancreatic Ductal Adenocarcinoma.Mol Cell Biol. 2022 Jul 21;42(7):e0001822. doi: 10.1128/mcb.00018-22. Epub 2022 Jun 15. Mol Cell Biol. 2022. PMID: 35703534 Free PMC article.
-
Cancer cell-mitochondria hybrid membrane coated Gboxin loaded nanomedicines for glioblastoma treatment.Nat Commun. 2023 Jul 28;14(1):4557. doi: 10.1038/s41467-023-40280-3. Nat Commun. 2023. PMID: 37507371 Free PMC article.
-
The RNA-binding protein MEX3A is a prognostic factor and regulator of resistance to gemcitabine in pancreatic ductal adenocarcinoma.Mol Oncol. 2021 Feb;15(2):579-595. doi: 10.1002/1878-0261.12847. Epub 2020 Nov 24. Mol Oncol. 2021. PMID: 33159833 Free PMC article.
-
Strategies for the Management of Patients with Pancreatic Cancer with PARP Inhibitors.Cancer Treat Res. 2023;186:125-142. doi: 10.1007/978-3-031-30065-3_8. Cancer Treat Res. 2023. PMID: 37978134
References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014;74(11):2913–21. - PubMed
-
- Society AC. American Cancer Society: Cancer Facts and Figures. 2015 http://wwwcancerorg/research/cancerfactsfigures/index.
-
- Carnevale J, Ashworth A. Assessing the Significance of BRCA1 and BRCA2 Mutations in Pancreatic Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(28):3080–1. - PubMed
-
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7. - PubMed
-
- Forster MD, Dedes KJ, Sandhu S, Frentzas S, Kristeleit R, Ashworth A, et al. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nature reviews Clinical oncology. 2011;8(5):302–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

