Proinflammatory CXCL12-CXCR4/CXCR7 Signaling Axis Drives Myc-Induced Prostate Cancer in Obese Mice
- PMID: 28687617
- PMCID: PMC5600849
- DOI: 10.1158/0008-5472.CAN-17-0284
Proinflammatory CXCL12-CXCR4/CXCR7 Signaling Axis Drives Myc-Induced Prostate Cancer in Obese Mice
Abstract
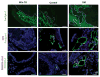
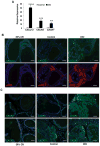
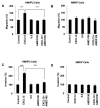
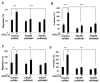
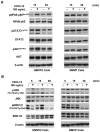
Obesity is a prognostic risk factor in the progression of prostate cancer; however, the molecular mechanisms involved are unclear. In this study, we provide preclinical proof of concept for the role of a proinflammatory CXCL12-CXCR4/CXCR7 signaling axis in an obesity-driven mouse model of myc-induced prostate cancer. Analysis of the stromal vascular fraction from periprostatic white adipose tissue from obese HiMyc mice at 6 months of age revealed a dramatic increase in mRNAs encoding various chemokines, cytokines, growth factors, and angiogenesis mediators, with CXCL12 among the most significantly upregulated genes. Immunofluorescence staining of ventral prostate tissue from obese HiMyc mice revealed high levels of CXCL12 in the stromal compartment as well as high staining for CXCR4 and CXCR7 in the epithelial compartment of tumors. Prostate cancer cell lines derived from HiMyc tumors (HMVP2 and derivative cell lines) displayed increased protein expression of both CXCR4 and CXCR7 compared with protein lysates from a nontumorigenic prostate epithelial cell line (NMVP cells). CXCL12 treatment stimulated migration and invasion of HMVP2 cells but not NMVP cells. These effects of CXCL12 on HMVP2 cells were inhibited by the CXCR4 antagonist AMD3100 as well as knockdown of either CXCR4 or CXCR7. CXCL12 treatment also produced rapid activation of STAT3, NFκB, and MAPK signaling in HMVP2 cells, which was again attenuated by either AMD3100 or knockdown of CXCR4 or CXCR7. Collectively, these data suggest that CXCL12 secreted by stromal cells activates invasiveness of prostate cancer cells and may play a role in driving tumor progression in obesity. Targeting the CXCL12-CXCR4/CXCR7 axis could lead to novel approaches for offsetting the effects of obesity on prostate cancer progression. Cancer Res; 77(18); 5158-68. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures
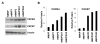
Similar articles
-
Inflammatory CXCL12-CXCR4/CXCR7 axis mediates G-protein signaling pathway to influence the invasion and migration of nasopharyngeal carcinoma cells.Tumour Biol. 2016 Jun;37(6):8169-79. doi: 10.1007/s13277-015-4686-2. Epub 2015 Dec 29. Tumour Biol. 2016. PMID: 26715277
-
Androgen receptor and chemokine receptors 4 and 7 form a signaling axis to regulate CXCL12-dependent cellular motility.BMC Cancer. 2015 Mar 31;15:204. doi: 10.1186/s12885-015-1201-5. BMC Cancer. 2015. PMID: 25884570 Free PMC article.
-
CXCR4 and CXCR7 signaling promotes tumor progression and obesity-associated epithelial-mesenchymal transition in prostate cancer cells.Oncogene. 2022 Oct;41(41):4633-4644. doi: 10.1038/s41388-022-02466-9. Epub 2022 Sep 10. Oncogene. 2022. PMID: 36088505
-
CXCL12-CXCR4/CXCR7 Axis in Colorectal Cancer: Therapeutic Target in Preclinical and Clinical Studies.Int J Mol Sci. 2021 Jul 9;22(14):7371. doi: 10.3390/ijms22147371. Int J Mol Sci. 2021. PMID: 34298991 Free PMC article. Review.
-
Drug Design Targeting the CXCR4/CXCR7/CXCL12 Pathway.Curr Top Med Chem. 2016;16(13):1441-51. doi: 10.2174/1568026615666150915120218. Curr Top Med Chem. 2016. PMID: 26369824 Review.
Cited by
-
Harnessing function of EMT in cancer drug resistance: a metastasis regulator determines chemotherapy response.Cancer Metastasis Rev. 2024 Mar;43(1):457-479. doi: 10.1007/s10555-023-10162-7. Epub 2024 Jan 16. Cancer Metastasis Rev. 2024. PMID: 38227149 Review.
-
Metabolomics-based phenotypic screens for evaluation of drug synergy via direct-infusion mass spectrometry.iScience. 2022 Apr 7;25(5):104221. doi: 10.1016/j.isci.2022.104221. eCollection 2022 May 20. iScience. 2022. PMID: 35494234 Free PMC article.
-
White adipose tissue-derived factors and prostate cancer progression: mechanisms and targets for interventions.Cancer Metastasis Rev. 2022 Sep;41(3):649-671. doi: 10.1007/s10555-022-10056-0. Epub 2022 Aug 4. Cancer Metastasis Rev. 2022. PMID: 35927363 Free PMC article. Review.
-
CXCR7 Reactivates ERK Signaling to Promote Resistance to EGFR Kinase Inhibitors in NSCLC.Cancer Res. 2019 Sep 1;79(17):4439-4452. doi: 10.1158/0008-5472.CAN-19-0024. Epub 2019 Jul 4. Cancer Res. 2019. PMID: 31273063 Free PMC article.
-
Inhibition of the CXCL12/CXCR4 axis prevents periurethral collagen accumulation and lower urinary tract dysfunction in vivo.Prostate. 2019 May;79(7):757-767. doi: 10.1002/pros.23781. Epub 2019 Feb 27. Prostate. 2019. PMID: 30811623 Free PMC article.
References
-
- Nomura AM. Body size and prostate cancer. Epidemiol Rev. 2001;23:126–31. - PubMed
-
- Porter MP, Stanford JL. Obesity and the risk of prostate cancer. Prostate. 2005;62:316–21. - PubMed
-
- Spitz MR, Strom SS, Yamamura Y, Troncoso P, Babaian RJ, Scardino PT, et al. Epidemiologic determinants of clinically relevant prostate cancer. Int J Cancer. 2000;89:259–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

