Cancer-Associated Fibroblasts Share Characteristics and Protumorigenic Activity with Mesenchymal Stromal Cells
- PMID: 28687621
- PMCID: PMC5600847
- DOI: 10.1158/0008-5472.CAN-16-2586
Cancer-Associated Fibroblasts Share Characteristics and Protumorigenic Activity with Mesenchymal Stromal Cells
Abstract
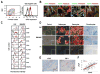
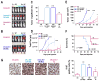
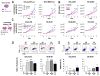
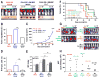
Cancer-associated fibroblasts (CAF) have been suggested to originate from mesenchymal stromal cells (MSC), but their relationship with MSCs is not clear. Here, we have isolated from primary human neuroblastoma tumors a population of αFAP- and FSP-1-expressing CAFs that share phenotypic and functional characteristics with bone marrow-derived MSCs (BM-MSC). Analysis of human neuroblastoma tumors also confirmed the presence of αFAP- and FSP-1-positive cells in the tumor stroma, and their presence correlated with that of M2 tumor-associated macrophages. These cells (designated CAF-MSCs) enhanced in vitro neuroblastoma cell proliferation, survival, and resistance to chemotherapy and stimulated neuroblastoma tumor engraftment and growth in immunodeficient mice, indicating an effect independent of the immune system. The protumorigenic activity of MSCs in vitro and in xenografted mice was dependent on the coactivation of JAK2/STAT3 and MEK/ERK1/2 in neuroblastoma cells. In a mouse model of orthotopically implanted neuroblastoma cells, inhibition of JAK2/STAT3 and MEK/ERK/1/2 by ruxolitinib and trametinib potentiated tumor response to etoposide and increased overall survival. These data point to a new type of protumorigenic CAF in the tumor microenvironment of neuroblastoma and to STAT3 and ERK1/2 as mediators of their activity. Cancer Res; 77(18); 5142-57. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures



References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. - PubMed
-
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. NatMed. 2001;7:1194–201. - PubMed
-
- Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol. 2013;33:1478–83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

