Clock1a affects mesoderm development and primitive hematopoiesis by regulating Nodal-Smad3 signaling in the zebrafish embryo
- PMID: 28687631
- PMCID: PMC5572899
- DOI: 10.1074/jbc.M117.794289
Clock1a affects mesoderm development and primitive hematopoiesis by regulating Nodal-Smad3 signaling in the zebrafish embryo
Abstract
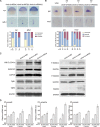
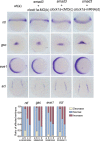
Circadian clock and Smad2/3/4-mediated Nodal signaling regulate multiple physiological and pathological processes. However, it remains unknown whether Clock directly cross-talks with Nodal signaling and how this would regulate embryonic development. Here we show that Clock1a coordinated mesoderm development and primitive hematopoiesis in zebrafish embryos by directly up-regulating Nodal-Smad3 signaling. We found that Clock1a is expressed both maternally and zygotically throughout early zebrafish development. We also noted that Clock1a alterations produce embryonic defects with shortened body length, lack of the ventral tail fin, or partial defect of the eyes. Clock1a regulates the expression of the mesodermal markers ntl, gsc, and eve1 and of the hematopoietic markers scl, lmo2, and fli1a Biochemical analyses revealed that Clock1a stimulates Nodal signaling by increasing expression of Smad2/3/4. Mechanistically, Clock1a activates the smad3a promoter via its E-box1 element (CAGATG). Taken together, these findings provide mechanistic insight into the role of Clock1a in the regulation of mesoderm development and primitive hematopoiesis via modulation of Nodal-Smad3 signaling and indicate that Smad3a is directly controlled by the circadian clock in zebrafish.
Keywords: SMAD transcription factor; clock gene; hematopoiesis; mesoderm; nodal; transcription promoter; zebrafish.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






Similar articles
-
Maternal Eomesodermin regulates zygotic nodal gene expression for mesendoderm induction in zebrafish embryos.J Mol Cell Biol. 2014 Aug;6(4):272-85. doi: 10.1093/jmcb/mju028. Epub 2014 Jun 12. J Mol Cell Biol. 2014. PMID: 24924767
-
smad2 and smad3 are required for mesendoderm induction by transforming growth factor-beta/nodal signals in zebrafish.J Biol Chem. 2008 Jan 25;283(4):2418-26. doi: 10.1074/jbc.M707578200. Epub 2007 Nov 19. J Biol Chem. 2008. PMID: 18025082
-
[Rbb4l enhances TGF-β/Nodal signaling and promotes zebrafish embryonic dorsolization].Yi Chuan. 2013 Apr;35(4):477-87. doi: 10.3724/sp.j.1005.2013.00477. Yi Chuan. 2013. PMID: 23659938 Review. Chinese.
-
ETV6 (TEL1) regulates embryonic hematopoiesis in zebrafish.Haematologica. 2015 Jan;100(1):23-31. doi: 10.3324/haematol.2014.104091. Epub 2014 Oct 3. Haematologica. 2015. PMID: 25281506 Free PMC article.
-
Patterning of the zebrafish embryo by nodal signals.Curr Top Dev Biol. 2003;55:143-71. doi: 10.1016/s0070-2153(03)01003-2. Curr Top Dev Biol. 2003. PMID: 12959195 Review. No abstract available.
Cited by
-
Gain of gene regulatory network interconnectivity at the origin of vertebrates.Proc Natl Acad Sci U S A. 2022 Mar 15;119(11):e2114802119. doi: 10.1073/pnas.2114802119. Epub 2022 Mar 9. Proc Natl Acad Sci U S A. 2022. PMID: 35263228 Free PMC article.
-
Adaptation and convergence in circadian-related genes in Iberian freshwater fish.BMC Ecol Evol. 2021 Mar 8;21(1):38. doi: 10.1186/s12862-021-01767-z. BMC Ecol Evol. 2021. PMID: 33685402 Free PMC article.
-
The Lineage Before Time: Circadian and Nonclassical Clock Influences on Development.Annu Rev Cell Dev Biol. 2020 Oct 6;36:469-509. doi: 10.1146/annurev-cellbio-100818-125454. Annu Rev Cell Dev Biol. 2020. PMID: 33021821 Free PMC article. Review.
-
The contribution of circadian clock to the biological processes.Front Mol Biosci. 2024 Jun 6;11:1387576. doi: 10.3389/fmolb.2024.1387576. eCollection 2024. Front Mol Biosci. 2024. PMID: 38903177 Free PMC article. Review.
-
Exploring associations of maternal sleep during periconceptional period with congenital heart disease in offspring.Birth Defects Res. 2019 Aug 1;111(13):920-931. doi: 10.1002/bdr2.1536. Epub 2019 Jun 17. Birth Defects Res. 2019. PMID: 31206252 Free PMC article.
References
-
- Bosler O., Girardet C., Franc J. L., Becquet D., and François-Bellan A. M. (2015) Structural plasticity of the circadian timing system: an overview from flies to mammals. Front. Neuroendocrinol. 38, 50–64 - PubMed
-
- Albrecht U. (2012) Timing to perfection: the biology of central and peripheral circadian clocks. Neuron. 74, 246–260 - PubMed
-
- Isorna E., de Pedro N., Valenciano A. I., Alonso-Gómez Á. L., Delgado M. J. (2017) Interplay between the endocrine and circadian systems in fishes. J. Endocrinol. 232, R141–R159 - PubMed
-
- Zhang L., Weng W., and Guo J. (2011) Posttranscriptional mechanisms in controlling eukaryotic circadian rhythms. FEBS Lett. 585, 1400–1405 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

