Antigen Acquisition Enables Newly Arriving B Cells To Enter Ongoing Immunization-Induced Germinal Centers
- PMID: 28687657
- PMCID: PMC5548600
- DOI: 10.4049/jimmunol.1700267
Antigen Acquisition Enables Newly Arriving B Cells To Enter Ongoing Immunization-Induced Germinal Centers
Abstract
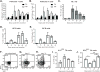
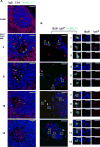
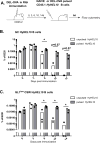
Modern vaccines must be designed to generate long-lasting, high-affinity, and broadly neutralizing Ab responses against pathogens. The diversity of B cell clones recruited into germinal center (GC) responses is likely to be important for the Ag-neutralization potential of the Ab-secreting cells and memory cells generated upon immunization. However, the factors that influence the diversity of B cell clones recruited into GCs are unclear. As recirculating naive Ag-specific B cells arrive in Ag-draining secondary lymphoid organs, they may join the ongoing GC response. However, the factors that limit their entry are not well understood, and it is not known how that depends on the stage of the ongoing follicular T cell and GC B cell response. In this article, we show that, in mice, naive B cells have a limited window of time during which they can undergo Ag-driven activation and join ongoing immunization-induced GC responses. However, preloading naive B cells with even a threshold-activating amount of Ag is sufficient to rescue their entry into the GC response during its initiation, peak, and contraction. Based on these results, we suggest that productive acquisition of Ag may be one of the main factors limiting entry of new B cell clones into ongoing immunization-triggered GC responses.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures

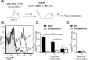
Similar articles
-
Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination.J Immunol. 2015 Aug 15;195(4):1617-27. doi: 10.4049/jimmunol.1402604. Epub 2015 Jul 13. J Immunol. 2015. PMID: 26170383
-
Protective Humoral Immunity in the Central Nervous System Requires Peripheral CD19-Dependent Germinal Center Formation following Coronavirus Encephalomyelitis.J Virol. 2017 Nov 14;91(23):e01352-17. doi: 10.1128/JVI.01352-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931676 Free PMC article.
-
Role of MHC class II on memory B cells in post-germinal center B cell homeostasis and memory response.J Immunol. 2006 Feb 15;176(4):2122-33. doi: 10.4049/jimmunol.176.4.2122. J Immunol. 2006. PMID: 16455968
-
The germinal center reaction.J Allergy Clin Immunol. 2010 Nov;126(5):898-907; quiz 908-9. doi: 10.1016/j.jaci.2010.09.007. J Allergy Clin Immunol. 2010. PMID: 21050940 Review.
-
Germinal center B-cells.Autoimmunity. 2012 Aug;45(5):333-47. doi: 10.3109/08916934.2012.665524. Epub 2012 Apr 2. Autoimmunity. 2012. PMID: 22390182 Review.
Cited by
-
Control of Germinal Center Responses by T-Follicular Regulatory Cells.Front Immunol. 2018 Aug 24;9:1910. doi: 10.3389/fimmu.2018.01910. eCollection 2018. Front Immunol. 2018. PMID: 30197643 Free PMC article. Review.
-
When designing vaccines, consider the starting material: the human B cell repertoire.Curr Opin Immunol. 2018 Aug;53:209-216. doi: 10.1016/j.coi.2018.08.002. Epub 2018 Sep 3. Curr Opin Immunol. 2018. PMID: 30190230 Free PMC article. Review.
-
Germinal center entry not selection of B cells is controlled by peptide-MHCII complex density.Nat Commun. 2018 Mar 2;9(1):928. doi: 10.1038/s41467-018-03382-x. Nat Commun. 2018. PMID: 29500348 Free PMC article.
-
Continuous germinal center invasion contributes to the diversity of the immune response.Cell. 2023 Jan 5;186(1):147-161.e15. doi: 10.1016/j.cell.2022.11.032. Epub 2022 Dec 23. Cell. 2023. PMID: 36565698 Free PMC article.
-
Diverse priming outcomes under conditions of very rare precursor B cells.Immunity. 2025 Apr 8;58(4):997-1014.e11. doi: 10.1016/j.immuni.2025.03.003. Epub 2025 Mar 31. Immunity. 2025. PMID: 40168992 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

