Mutation of the α-tubulin Tuba1a leads to straighter microtubules and perturbs neuronal migration
- PMID: 28687665
- PMCID: PMC5551700
- DOI: 10.1083/jcb.201607074
Mutation of the α-tubulin Tuba1a leads to straighter microtubules and perturbs neuronal migration
Abstract
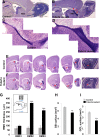
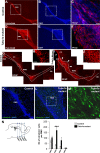
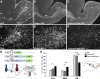
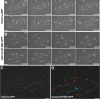
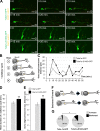
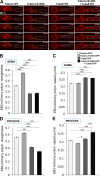
Brain development involves extensive migration of neurons. Microtubules (MTs) are key cellular effectors of neuronal displacement that are assembled from α/β-tubulin heterodimers. Mutation of the α-tubulin isotype TUBA1A is associated with cortical malformations in humans. In this study, we provide detailed in vivo and in vitro analyses of Tuba1a mutants. In mice carrying a Tuba1a missense mutation (S140G), neurons accumulate, and glial cells are dispersed along the rostral migratory stream in postnatal and adult brains. Live imaging of Tuba1a-mutant neurons revealed slowed migration and increased neuronal branching, which correlated with directionality alterations and perturbed nucleus-centrosome (N-C) coupling. Tuba1a mutation led to increased straightness of newly polymerized MTs, and structural modeling data suggest a conformational change in the α/β-tubulin heterodimer. We show that Tuba8, another α-tubulin isotype previously associated with cortical malformations, has altered function compared with Tuba1a. Our work shows that Tuba1a plays an essential, noncompensated role in neuronal saltatory migration in vivo and highlights the importance of MT flexibility in N-C coupling and neuronal-branching regulation during neuronal migration.
© 2017 Belvindrah et al.
Figures


Comment in
-
Tubulin isotype specificity in neuronal migration: Tuba8 can't fill in for Tuba1a.J Cell Biol. 2017 Aug 7;216(8):2247-2249. doi: 10.1083/jcb.201705172. Epub 2017 Jul 7. J Cell Biol. 2017. PMID: 28687668 Free PMC article.
-
Tubulin diversity and neuronal migration.Cell Cycle. 2018;17(4):405-406. doi: 10.1080/15384101.2018.1439822. Epub 2018 Apr 3. Cell Cycle. 2018. PMID: 29460664 Free PMC article. No abstract available.
References
-
- Abdollahi M.R., Morrison E., Sirey T., Molnar Z., Hayward B.E., Carr I.M., Springell K., Woods C.G., Ahmed M., Hattingh L., et al. . 2009. Mutation of the variant α-tubulin TUBA8 results in polymicrogyria with optic nerve hypoplasia. Am. J. Hum. Genet. 85:737–744. 10.1016/j.ajhg.2009.10.007 - DOI - PMC - PubMed
-
- Bahi-Buisson N., and Cavallin M.. 2016. Tubulinopathies Overview. In GeneReviews. Pagon R.A., Adam M.P., Ardinger H.H., Wallace S.E., Amemiya A., Bean L.J.H., Bird T.D., Ledbetter N., Mefford H.C., Smith R.J.H., and Stephens K., editors. University of Washington, Seattle: Available at: https://www.ncbi.nlm.nih.gov/books/NBK350554/ - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

