The Optimal Timing of Stage 2 Palliation for Hypoplastic Left Heart Syndrome: An Analysis of the Pediatric Heart Network Single Ventricle Reconstruction Trial Public Data Set
- PMID: 28687711
- PMCID: PMC5664211
- DOI: 10.1161/CIRCULATIONAHA.117.028481
The Optimal Timing of Stage 2 Palliation for Hypoplastic Left Heart Syndrome: An Analysis of the Pediatric Heart Network Single Ventricle Reconstruction Trial Public Data Set
Abstract
Background: In infants requiring 3-stage single-ventricle palliation for hypoplastic left heart syndrome, attrition after the Norwood procedure remains significant. The effect of the timing of stage 2 palliation (S2P), a physician-modifiable factor, on long-term survival is not well understood. We hypothesized that an optimal interval between the Norwood and S2P that both minimizes pre-S2P attrition and maximizes post-S2P survival exists and is associated with individual patient characteristics.
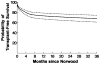
Methods: The National Institutes of Health/National Heart, Lung, and Blood Institute Pediatric Heart Network Single Ventricle Reconstruction Trial public data set was used. Transplant-free survival (TFS) was modeled from (1) Norwood to S2P and (2) S2P to 3 years by using parametric hazard analysis. Factors associated with death or heart transplantation were determined for each interval. To account for staged procedures, risk-adjusted, 3-year, post-Norwood TFS (the probability of TFS at 3 years given survival to S2P) was calculated using parametric conditional survival analysis. TFS from the Norwood to S2P was first predicted. TFS after S2P to 3 years was then predicted and adjusted for attrition before S2P by multiplying by the estimate of TFS to S2P. The optimal timing of S2P was determined by generating nomograms of risk-adjusted, 3-year, post-Norwood, TFS versus the interval from the Norwood to S2P.
Results: Of 547 included patients, 399 survived to S2P (73%). Of the survivors to S2P, 349 (87%) survived to 3-year follow-up. The median interval from the Norwood to S2P was 5.1 (interquartile range, 4.1-6.0) months. The risk-adjusted, 3-year, TFS was 68±7%. A Norwood-S2P interval of 3 to 6 months was associated with greatest 3-year TFS overall and in patients with few risk factors. In patients with multiple risk factors, TFS was severely compromised, regardless of the timing of S2P and most severely when S2P was performed early. No difference in the optimal timing of S2P existed when stratified by shunt type.
Conclusions: In infants with few risk factors, progressing to S2P at 3 to 6 months after the Norwood procedure was associated with maximal TFS. Early S2P did not rescue patients with greater risk factor burdens. Instead, referral for heart transplantation may offer their best chance at long-term survival.
Clinical trial registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT00115934.
Keywords: arteriovenous shunt, surgical; heart defects, congenital; statistics; surgery; survival.
© 2017 American Heart Association, Inc.
Figures





References
-
- Ghanayem NS, Tweddell JS, Hoffman GM, Mussatto K, Jaquiss RD. Optimal timing of the second stage of palliation for hypoplastic left heart syndrome facilitated through home monitoring, and the results of early cavopulmonary anastomosis. Cardiol young. 2006;16(Suppl 1):61–66. - PubMed
-
- Hehir DA, Dominguez TE, Ballweg JA, Ravishankar C, Marino BS, Bird GL, Nicolson SC, Spray TL, Gaynor JW, Tabbutt S. Risk factors for interstage death after stage 1 reconstruction of hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg. 2008;136:94–99. 99 e91–93. - PubMed
-
- Simsic JM, Bradley SM, Stroud MR, Atz AM. Risk factors for interstage death after the norwood procedure. Pediatr Cardiol. 2005;26:400–403. - PubMed
-
- Ohye RG, Schonbeck JV, Eghtesady P, Laussen PC, Pizarro C, Shrader P, Frank DU, Graham EM, Hill KD, Jacobs JP, Kanter KR, Kirsh JA, Lambert LM, Lewis AB, Ravishankar C, Tweddell JS, Williams IA, Pearson GD Pediatric Heart Network I. Cause, timing, and location of death in the single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:907–914. - PMC - PubMed
-
- Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, Eghtesady P, Frommelt PC, Gruber PJ, Hill KD, Kaltman JR, Laussen PC, Lewis AB, Lurito KJ, Minich LL, Ohye RG, Schonbeck JV, Schwartz SM, Singh RK, Goldberg CS Pediatric Heart Network I. Interstage mortality after the norwood procedure: Results of the multicenter single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

