Lamin B1 levels modulate differentiation into neurons during embryonic corticogenesis
- PMID: 28687747
- PMCID: PMC5501862
- DOI: 10.1038/s41598-017-05078-6
Lamin B1 levels modulate differentiation into neurons during embryonic corticogenesis
Abstract
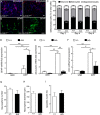
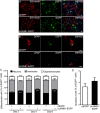
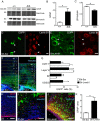
Lamin B1, a key component of the nuclear lamina, plays an important role in brain development. Ablation of endogenous Lamin B1 (Lmnb1) in the mouse strongly impairs embryonic brain development and corticogenesis. However, the mechanisms underlying these neurodevelopmental effects are unknown. Here, we report that Lamin B1 levels modulate the differentiation of murine neural stem cells (NSCs) into neurons and astroglial-like cells. In vitro, endogenous Lmnb1 depletion favors NSC differentiation into glial fibrillar acidic protein (GFAP)-immunoreactive cells over neurons, while overexpression of human Lamin B1 (LMNB1) increases the proportion of neurons. In Lmnb1-null embryos, neurogenesis is reduced, while in vivo Lmnb1 silencing in mouse embryonic brain by in utero electroporation of a specific Lmnb1 sh-RNA results in aberrant cortical positioning of neurons and increased expression of the astrocytic marker GFAP in the cortex of 7-day old pups. Together, these results indicate that finely tuned levels of Lamin B1 are required for NSC differentiation into neurons, proper expression of the astrocytic marker GFAP and corticogenesis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Worman HJ, Lazaridis I, Georgatos SD. Nuclear lamina heterogeneity in mammalian cells. Differential expression of the major lamins and variations in lamin B phosphorylation. J. Biol. Chem. 1988;263:12135–12141. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

