The interaction of the bioinsecticide PA1b (Pea Albumin 1 subunit b) with the insect V-ATPase triggers apoptosis
- PMID: 28687751
- PMCID: PMC5501856
- DOI: 10.1038/s41598-017-05315-y
The interaction of the bioinsecticide PA1b (Pea Albumin 1 subunit b) with the insect V-ATPase triggers apoptosis
Abstract
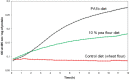
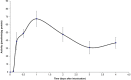
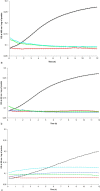
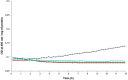
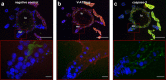
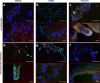
PA1b (Pea Albumin 1, subunit b) peptide is an entomotoxin, extracted from Legume seeds, with a lethal activity towards several insect pests, such as mosquitoes, some aphids and cereal weevils. This toxin acts by binding to the subunits c and e of the plasma membrane H+-ATPase (V-ATPase) in the insect midgut. In this study, two cereal weevils, the sensitive Sitophilus oryzae strain WAA42, the resistance Sitophilus oryzae strain ISOR3 and the insensitive red flour beetle Tribolium castaneum, were used in biochemical and histological experiments to demonstrate that a PA1b/V-ATPase interaction triggers the apoptosis mechanism, resulting in insect death. Upon intoxication with PA1b, apoptotic bodies are formed in the cells of the insect midgut. In addition, caspase-3 enzyme activity occurs in the midgut of sensitive weevils after intoxication with active PA1b, but not in the midgut of resistant weevils. These biochemical data were confirmed by immuno-histochemical detection of the caspase-3 active form in the midgut of sensitive weevils. Immuno-labelling experiments also revealed that the caspase-3 active form and V-ATPase are close-localized in the insect midgut. The results concerning this unique peptidic V-ATPase inhibitor pave the way for the utilization of PA1b as a promising, more selective and eco-friendly insecticide.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
New mode of action for a knottin protein bioinsecticide: pea albumin 1 subunit b (PA1b) is the first peptidic inhibitor of V-ATPase.J Biol Chem. 2011 Oct 21;286(42):36291-6. doi: 10.1074/jbc.M111.281055. Epub 2011 Sep 2. J Biol Chem. 2011. PMID: 21890633 Free PMC article.
-
Expression and biological activity of the cystine knot bioinsecticide PA1b (Pea Albumin 1 Subunit b).PLoS One. 2013 Dec 11;8(12):e81619. doi: 10.1371/journal.pone.0081619. eCollection 2013. PLoS One. 2013. PMID: 24349099 Free PMC article.
-
Host range of the potential biopesticide Pea Albumin 1b (PA1b) is limited to insects.Toxicon. 2014 Oct;89:67-76. doi: 10.1016/j.toxicon.2014.07.004. Epub 2014 Jul 23. Toxicon. 2014. PMID: 25064271
-
Pea Albumin 1 subunit b (PA1b), a promising bioinsecticide of plant origin.Toxins (Basel). 2011 Dec;3(12):1502-17. doi: 10.3390/toxins3121502. Epub 2011 Dec 8. Toxins (Basel). 2011. PMID: 22295174 Free PMC article. Review.
-
Regulation of V-ATPase activity.Front Biosci (Landmark Ed). 2017 Jan 1;22(4):609-622. doi: 10.2741/4506. Front Biosci (Landmark Ed). 2017. PMID: 27814636 Review.
Cited by
-
Insect Pest Control from Chemical to Biotechnological Approach: Constrains and Challenges.Insects. 2025 May 15;16(5):528. doi: 10.3390/insects16050528. Insects. 2025. PMID: 40429241 Free PMC article. Review.
-
Nutritional and Pharmaceutical Applications of Under-Explored Knottin Peptide-Rich Phytomedicines.Plants (Basel). 2022 Nov 28;11(23):3271. doi: 10.3390/plants11233271. Plants (Basel). 2022. PMID: 36501311 Free PMC article. Review.
-
Gene Sequences of Potential Targets of Insecticidal PF2 Lectin Identified from the Larval De Novo Transcriptome of the Mexican Bean Weevil (Zabrotes Subfasciatus; Boheman 1833).Insects. 2020 Oct 27;11(11):736. doi: 10.3390/insects11110736. Insects. 2020. PMID: 33121035 Free PMC article.
-
Residues of Legume AG41 Peptide Crucial to Its Bio-Insecticidal Activity.Biomolecules. 2023 Feb 27;13(3):446. doi: 10.3390/biom13030446. Biomolecules. 2023. PMID: 36979381 Free PMC article.
-
The Archetypal Gamma-Core Motif of Antimicrobial Cys-Rich Peptides Inhibits H+-ATPases in Target Pathogens.Int J Mol Sci. 2024 Sep 6;25(17):9672. doi: 10.3390/ijms25179672. Int J Mol Sci. 2024. PMID: 39273619 Free PMC article.
References
-
- Delobel, B., Grenier, A. M., Gueguen, J., Ferrasson, E. & Mbaiguinam, M. Utilisation d’un polypeptide dérivé d’une albumine PA1b de légumineuse comme insecticide (Brevet-98/05877). Paris patent (1998).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

