Efficient activation of the lymphangiogenic growth factor VEGF-C requires the C-terminal domain of VEGF-C and the N-terminal domain of CCBE1
- PMID: 28687807
- PMCID: PMC5501841
- DOI: 10.1038/s41598-017-04982-1
Efficient activation of the lymphangiogenic growth factor VEGF-C requires the C-terminal domain of VEGF-C and the N-terminal domain of CCBE1
Abstract
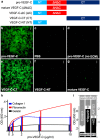
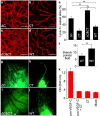
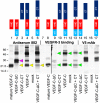
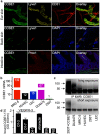
The collagen- and calcium-binding EGF domains 1 (CCBE1) protein is necessary for lymphangiogenesis. Its C-terminal collagen-like domain was shown to be required for the activation of the major lymphangiogenic growth factor VEGF-C (Vascular Endothelial Growth Factor-C) along with the ADAMTS3 (A Disintegrin And Metalloproteinase with Thrombospondin Motifs-3) protease. However, it remained unclear how the N-terminal domain of CCBE1 contributed to lymphangiogenic signaling. Here, we show that efficient activation of VEGF-C requires its C-terminal domain both in vitro and in a transgenic mouse model. The N-terminal EGF-like domain of CCBE1 increased VEGFR-3 signaling by colocalizing pro-VEGF-C with its activating protease to the lymphatic endothelial cell surface. When the ADAMTS3 amounts were limited, proteolytic activation of pro-VEGF-C was supported by the N-terminal domain of CCBE1, but not by its C-terminal domain. A single amino acid substitution in ADAMTS3, identified from a lymphedema patient, was associated with abnormal CCBE1 localization. These results show that CCBE1 promotes VEGFR-3 signaling and lymphangiogenesis by different mechanisms, which are mediated independently by the two domains of CCBE1: by enhancing the cleavage activity of ADAMTS3 and by facilitating the colocalization of VEGF-C and ADAMTS3. These new insights should be valuable in developing new strategies to therapeutically target VEGF-C/VEGFR-3-induced lymphangiogenesis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD.J Clin Invest. 2016 Jun 1;126(6):2167-80. doi: 10.1172/JCI83967. Epub 2016 May 9. J Clin Invest. 2016. PMID: 27159393 Free PMC article.
-
CCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation.Circulation. 2014 May 13;129(19):1962-71. doi: 10.1161/CIRCULATIONAHA.113.002779. Epub 2014 Feb 19. Circulation. 2014. PMID: 24552833
-
Functional Dissection of the CCBE1 Protein: A Crucial Requirement for the Collagen Repeat Domain.Circ Res. 2015 May 8;116(10):1660-9. doi: 10.1161/CIRCRESAHA.116.304949. Epub 2015 Mar 26. Circ Res. 2015. PMID: 25814692
-
Molecular targeting of lymphatics for therapy.Curr Pharm Des. 2004;10(1):65-74. doi: 10.2174/1381612043453513. Curr Pharm Des. 2004. PMID: 14754406 Review.
-
Roles of the TGF-β⁻VEGF-C Pathway in Fibrosis-Related Lymphangiogenesis.Int J Mol Sci. 2018 Aug 23;19(9):2487. doi: 10.3390/ijms19092487. Int J Mol Sci. 2018. PMID: 30142879 Free PMC article. Review.
Cited by
-
Engineered biomaterial strategies for controlling growth factors in tissue engineering.Drug Deliv. 2020 Dec;27(1):1438-1451. doi: 10.1080/10717544.2020.1831104. Drug Deliv. 2020. PMID: 33100031 Free PMC article. Review.
-
Lymphangiogenesis guidance by paracrine and pericellular factors.Genes Dev. 2017 Aug 15;31(16):1615-1634. doi: 10.1101/gad.303776.117. Genes Dev. 2017. PMID: 28947496 Free PMC article. Review.
-
The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease.Cell. 2020 Jul 23;182(2):270-296. doi: 10.1016/j.cell.2020.06.039. Cell. 2020. PMID: 32707093 Free PMC article. Review.
-
Visfatin Affects the Transcriptome of Porcine Luteal Cells during Early Pregnancy.Int J Mol Sci. 2024 Feb 16;25(4):2339. doi: 10.3390/ijms25042339. Int J Mol Sci. 2024. PMID: 38397019 Free PMC article.
-
Immune-interacting lymphatic endothelial subtype at capillary terminals drives lymphatic malformation.J Exp Med. 2023 Apr 3;220(4):e20220741. doi: 10.1084/jem.20220741. Epub 2023 Jan 23. J Exp Med. 2023. PMID: 36688917 Free PMC article.
References
-
- Krebs R, Jeltsch M. [The lymphangiogenic growth factors VEGF-C and VEGF-D Part 1: Basic principles and embryonic development] LymphForsch. 2013;17:30–37.
-
- Krebs R, Jeltsch M. [The lymphangiogenic growth factors VEGF-C and VEGF-D Part 2: The role of lymphangiogenic growth factors VEGF-C and VEGF-D in lymphatic disorders] LymphForsch. 2013;17:96–104.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

