Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients With Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial
- PMID: 28687830
- PMCID: PMC5710193
- DOI: 10.1001/jamaoncol.2017.1598
Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients With Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial
Erratum in
-
Incorrect Trial Database Number.JAMA Oncol. 2017 Nov 1;3(11):1589. doi: 10.1001/jamaoncol.2017.3950. JAMA Oncol. 2017. PMID: 29049461 Free PMC article. No abstract available.
Abstract
Importance: The role of epidermal growth factor receptor (EGFR) inhibition in chemoradiation strategies in the nonoperative treatment of patients with esophageal cancer remains uncertain.
Objective: To evaluate the benefit of cetuximab added to concurrent chemoradiation therapy for patients undergoing nonoperative treatment of esophageal carcinoma.
Design, setting, and participants: A National Cancer Institute (NCI) sponsored, multicenter, phase 3, randomized clinical trial open to patients with biopsy-proven carcinoma of the esophagus. The study accrued 344 patients from 2008 to 2013.
Interventions: Patients were randomized to weekly concurrent cisplatin (50 mg/m2), paclitaxel (25 mg/m2), and daily radiation of 50.4 Gy/1.8 Gy fractions with or without weekly cetuximab (400 mg/m2 on day 1 then 250 mg/m2 weekly).
Main outcomes and measures: Overall survival (OS) was the primary endpoint, with a study designed to detect an increase in 2-year OS from 41% to 53%; 80% power and 1-sided α = .025.
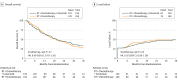
Results: Between June 30, 2008, and February 8, 2013, 344 patients were enrolled. This analysis used all data received at NRG Oncology through April 12, 2015. Sixteen patients were ineligible, resulting in 328 evaluable patients, 159 in the experimental arm and 169 in the control arm. Patients were well matched between the treatment arms for patient and tumor characteristics: 263 (80%) with T3 or T4 disease, 215 (66%) N1, and 62 (19%) with celiac nodal involvement. Incidence of grade 3, 4, or 5 treatment-related adverse events at any time was 71 (46%), 35 (23%), or 6 (4%) in the experimental arm and 83 (50%), 28 (17%), or 2 (1%) in the control arm, respectively. A clinical complete response (cCR) rate of 81 (56%) was observed in the experimental arm vs 92 (58%) in the control arm (Fisher exact test, P = .66). No differences were seen in cCR between treatment arms for either histology (adenocarcinoma or squamous cell). Median follow-up for all patients was 18.6 months. The 24- and 36-month local failure for the experimental arm was 47% (95% CI, 38%-57%) and 49% (95% CI, 40%-59%) vs 49% (95% CI, 41%-58%) and 49% (95% CI, 41%-58%) for the control arm (HR, 0.92; 95% CI, 0.66-1.28; P = .65). The 24- and 36-month OS rates for the experimental arm were 45% (95% CI, 37%-53%) and 34% (95% CI, 26%-41%) vs 44% (95% CI, 36%-51%) and 28% (95% CI, 21%-35%) for the control arm (HR, 0.90; 95% CI, 0.70-1.16; P = .47).
Conclusions and relevance: The addition of cetuximab to concurrent chemoradiation did not improve OS. These phase 3 trial results point to little benefit to current EGFR-targeted agents in an unselected patient population, and highlight the need for predictive biomarkers in the treatment of esophageal cancer.
Trial registration: clinicaltrials.gov Identifier: NCT00655876.
Conflict of interest statement
Figures
Comment in
-
Questions About a Clinical Trial Evaluating the Addition of Cetuximab to Definitive Chemoradiation Therapy With Paclitaxel and Cisplatin for Patients With Esophageal Cancer.JAMA Oncol. 2018 Jun 1;4(6):887-888. doi: 10.1001/jamaoncol.2018.0239. JAMA Oncol. 2018. PMID: 29596540 No abstract available.
-
Questions About a Clinical Trial Evaluating the Addition of Cetuximab to Definitive Chemoradiation Therapy With Paclitaxel and Cisplatin for Patients With Esophageal Cancer.JAMA Oncol. 2018 Jun 1;4(6):887. doi: 10.1001/jamaoncol.2018.0228. JAMA Oncol. 2018. PMID: 29596543 No abstract available.
-
Questions About a Clinical Trial Evaluating the Addition of Cetuximab to Definitive Chemoradiation Therapy With Paclitaxel and Cisplatin for Patients With Esophageal Cancer-Reply.JAMA Oncol. 2018 Jun 1;4(6):888-889. doi: 10.1001/jamaoncol.2018.0242. JAMA Oncol. 2018. PMID: 29596545 Free PMC article. No abstract available.
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29. - PubMed
-
- Cooper JS, Guo MD, Herskovic A, et al. ; Radiation Therapy Oncology Group . Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). JAMA. 1999;281(17):1623-1627. - PubMed
-
- Herskovic A, Martz K, al-Sarraf M, et al. . Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326(24):1593-1598. - PubMed
-
- Kelsen DP, Winter KA, Gunderson LL, et al. ; Radiation Therapy Oncology Group; USA Intergroup . Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25(24):3719-3725. - PubMed
-
- Minsky BD, Neuberg D, Kelsen DP, et al. . Final report of Intergroup Trial 0122 (ECOG PE-289, RTOG 90-12): Phase II trial of neoadjuvant chemotherapy plus concurrent chemotherapy and high-dose radiation for squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 1999;43(3):517-523. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous