Cellular mechanosensing of the biophysical microenvironment: A review of mathematical models of biophysical regulation of cell responses
- PMID: 28688729
- PMCID: PMC5712490
- DOI: 10.1016/j.plrev.2017.06.016
Cellular mechanosensing of the biophysical microenvironment: A review of mathematical models of biophysical regulation of cell responses
Abstract
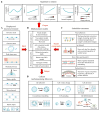
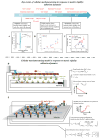
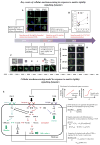
Cells in vivo reside within complex microenvironments composed of both biochemical and biophysical cues. The dynamic feedback between cells and their microenvironments hinges upon biophysical cues that regulate critical cellular behaviors. Understanding this regulation from sensing to reaction to feedback is therefore critical, and a large effort is afoot to identify and mathematically model the fundamental mechanobiological mechanisms underlying this regulation. This review provides a critical perspective on recent progress in mathematical models for the responses of cells to the biophysical cues in their microenvironments, including dynamic strain, osmotic shock, fluid shear stress, mechanical force, matrix rigidity, porosity, and matrix shape. The review highlights key successes and failings of existing models, and discusses future opportunities and challenges in the field.
Keywords: Biomechanics; Cellular mechanosensing; Focal adhesions; Mathematical modeling; Mechanobiology; Signaling pathway.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures




Comment in
-
Multiscale dynamics of the biophysical and biochemical microenvironment: Comment on "Cellular mechanosensing of the biophysical microenvironment: A review of mathematical models of biophysical regulation of cell responses" by Bo Cheng et al.Phys Life Rev. 2017 Dec;22-23:127-129. doi: 10.1016/j.plrev.2017.07.004. Epub 2017 Aug 1. Phys Life Rev. 2017. PMID: 28781238 No abstract available.
-
How can we predict cellular mechanosensation?: Comment on "Cellular mechanosensing of the biophysical microenvironment: A review of mathematical models of biophysical regulation of cell responses" by Bo Cheng et al.Phys Life Rev. 2017 Dec;22-23:120-122. doi: 10.1016/j.plrev.2017.08.013. Epub 2017 Sep 1. Phys Life Rev. 2017. PMID: 28890169 Free PMC article. No abstract available.
-
Cell adhesion mechanosensitivity, an active biological process: Comment on "Cellular mechanosensing of the biophysical microenvironment: A review of the mathematical models of biophysical regulation of cell responses" by Bo Cheng et al.Phys Life Rev. 2017 Dec;22-23:123-126. doi: 10.1016/j.plrev.2017.10.001. Epub 2017 Oct 7. Phys Life Rev. 2017. PMID: 29032920 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

