Patient navigation for lung cancer screening in an urban safety-net system: Protocol for a pragmatic randomized clinical trial
- PMID: 28689056
- PMCID: PMC7066861
- DOI: 10.1016/j.cct.2017.07.003
Patient navigation for lung cancer screening in an urban safety-net system: Protocol for a pragmatic randomized clinical trial
Abstract
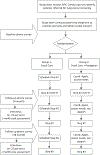
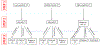
The National Lung Screening Trial demonstrated improved lung cancer mortality with annual low-dose computed tomography (CT) screening, leading to lung cancer screening endorsement by the United States Preventive Services Task Force and coverage by the Centers for Medicare and Medicaid. Adherence to annual CT screens in that trial was 95%, which may not be representative of real-world, particularly medically underserved populations. This pragmatic trial will determine the effect of patient-focused, telephone-based patient navigation on adherence to CT-based lung cancer screening in an urban safety-net population. 340 adults who meet standard eligibility for lung cancer screening (age 55-77years, smoking history≥30 pack-years, quit within 15years if former smoker) are referred through an electronic medical record-based order by physicians in community- and hospital-based primary care settings within the Parkland Health and Hospital System in Dallas County, Texas. Eligible patients are randomized to usual care or patient navigation, which addresses adherence, patient-reported barriers, smoking cessation, and psycho-social concerns related to screening completion. Patients complete surveys and semi-structured interviews at baseline, 6-month, and 18-month follow-ups to assess attitudes toward screening. The primary endpoint of this pragmatic trial is adherence to three sequential, prospectively defined steps in the screening protocol. Secondary endpoints include self-reported tobacco use and other patient-reported outcomes. Results will provide real-world insight into the impact of patient navigation on adherence to CT-based lung cancer screening in a medically underserved population. This study was registered with the NIH ClinicalTrials.gov database (NCT02758054) on April 26, 2016.
Keywords: Adherence; Lung cancer screening; Navigation; Patient reported outcomes; Pragmatic trial; Smoking cessation.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
References
-
- Cancer Facts & Figures 2013.
-
- Mulshine JL, Scott F, Molecular markers in early cancer detection. New screening tools, Chest 107 (6 Suppl) (1995) 280S–286S. - PubMed
-
- Hardy D, Xia R, Liu CC, Cormier JN, Nurgalieva Z, Du XL, Racial disparities and survival for nonsmall-cell lung cancer in a large cohort of black and white elderly patients, Cancer 115 (20) (2009) 4807–4818. - PubMed
-
- Bach PB, Cramer LD, Warren JL, Begg CB, Racial differences in the treatment of early-stage lung cancer, N. Engl. J. Med. 341 (16) (1999) 1198–1205. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

