Variation among Consent Forms for Clinical Whole Exome Sequencing
- PMID: 28689263
- PMCID: PMC5794809
- DOI: 10.1007/s10897-017-0127-2
Variation among Consent Forms for Clinical Whole Exome Sequencing
Abstract
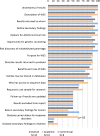
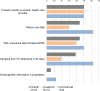
The goal of this study was to explore variation among informed consent documents for clinical whole exome sequencing (WES) in order to identify the level of consistency with the recommendations from the American College of Medical Genetics and Genomics (ACMG) and the Presidential Commission for the Study of Bioethical Issues (Bioethics Commission) regarding informed consent for clinical WES. Recommendations were organized into a framework of key points for analysis. Content analysis was conducted on a sample of informed consent documents for clinical WES downloaded from 18 laboratory websites. We observed considerable variability in the content of informed consent documents among the sample of 18 laboratories. The mean Flesch-Kincaid Grade Level, a measure of readability, of the consent forms was 10.8, above the recommended 8th grade level. For each of the individual ACMG and Bioethics Commission recommendations, the frequency of inclusion ranged from 11% to 100%. For the overall list of 18 consent items, inclusion ranged from 11 to 17 items (Mean = 13.44, Mode = 14). This analysis will be useful to laboratories that wish to create informed consent documents that comply with these recommendations. The consistent use of standardized informed consent process could improve communication between clinicians and patients and increase understanding of genetic testing.
Keywords: Bioethics; Exome sequencing; Informatics; Informed consent.
Conflict of interest statement
Funding
No external funding was utilized for this project.
Conflict of Interest
Sara A. Fowler, Carol J. Saunders, and Mark A. Hoffman declare that they have no conflict of interest.
Ethical Approval
This work was performed with documents that were publically available.
Human Studies and Informed Consent
No human subjects were involved in this descriptive analysis of public documents. No individual patient consents were reviewed.
Animal Studies
No animal studies were carried out by the authors for this article.
Figures
Similar articles
-
Shortened consent forms for genome-wide sequencing: Parent and provider perspectives.Mol Genet Genomic Med. 2020 Jul;8(7):e1254. doi: 10.1002/mgg3.1254. Epub 2020 May 8. Mol Genet Genomic Med. 2020. PMID: 32383361 Free PMC article.
-
The readability of informed consent forms for research studies conducted in South Africa.S Afr Med J. 2021 Feb 1;111(2):180-183. doi: 10.7196/SAMJ.2021.v111i2.14752. S Afr Med J. 2021. PMID: 33944731
-
Understanding variations in secondary findings reporting practices across U.S. genome sequencing laboratories.AJOB Empir Bioeth. 2018 Jan-Mar;9(1):48-57. doi: 10.1080/23294515.2017.1405095. Epub 2017 Dec 21. AJOB Empir Bioeth. 2018. PMID: 29131714
-
Whole Exome Sequencing: Applications in Prenatal Genetics.Obstet Gynecol Clin North Am. 2018 Mar;45(1):69-81. doi: 10.1016/j.ogc.2017.10.003. Obstet Gynecol Clin North Am. 2018. PMID: 29428287 Free PMC article. Review.
-
Improvement of informed consent and the quality of consent documents.Lancet Oncol. 2008 May;9(5):485-93. doi: 10.1016/S1470-2045(08)70128-1. Lancet Oncol. 2008. PMID: 18452859 Review.
Cited by
-
Consent for clinical genome sequencing: considerations from the Clinical Sequencing Exploratory Research Consortium.Per Med. 2019 Jul;16(4):325-333. doi: 10.2217/pme-2018-0076. Epub 2019 Jul 17. Per Med. 2019. PMID: 31313633 Free PMC article.
-
Analysis of informed consent forms of patients undergoing cancer genetic testing in the era of next-generation sequencing.Hered Cancer Clin Pract. 2025 Feb 21;23(1):8. doi: 10.1186/s13053-025-00309-8. Hered Cancer Clin Pract. 2025. PMID: 39985063 Free PMC article.
-
Shortened consent forms for genome-wide sequencing: Parent and provider perspectives.Mol Genet Genomic Med. 2020 Jul;8(7):e1254. doi: 10.1002/mgg3.1254. Epub 2020 May 8. Mol Genet Genomic Med. 2020. PMID: 32383361 Free PMC article.
-
The Clinical Sequencing Evidence-Generating Research Consortium: Integrating Genomic Sequencing in Diverse and Medically Underserved Populations.Am J Hum Genet. 2018 Sep 6;103(3):319-327. doi: 10.1016/j.ajhg.2018.08.007. Am J Hum Genet. 2018. PMID: 30193136 Free PMC article.
-
Assessment of the current status of real-world pharmacogenomic testing: informed consent, patient education, and related practices.Front Pharmacol. 2024 Feb 8;15:1355412. doi: 10.3389/fphar.2024.1355412. eCollection 2024. Front Pharmacol. 2024. PMID: 38410134 Free PMC article.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical