Factors affecting separation and detection of bile acids by liquid chromatography coupled with mass spectrometry in negative mode
- PMID: 28689325
- PMCID: PMC6554201
- DOI: 10.1007/s00216-017-0489-1
Factors affecting separation and detection of bile acids by liquid chromatography coupled with mass spectrometry in negative mode
Abstract
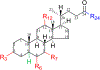
Bile acids (BAs) are cholesterol metabolites with important biological functions. They undergo extensive host-gut microbial co-metabolisms during the enterohepatic circulation, creating a vast structural diversity and resulting in great challenges to separate and detect them. Based on the bioanalytical reports in the past decade, this work developed three chromatographic gradient methods to separate a total of 48 BA standards on an ethylene-bridged hybrid (BEH) C18 column and high-strength silica (HSS) T3 column and accordingly unraveled the factors affecting the separation and detection of them by liquid chromatography coupled with mass spectrometry (LC-MS). It was shown that both the acidity and ammonium levels in mobile phases reduced the electrospray ionization (ESI) of BAs as anions of [M-H]-, especially for those unconjugated ones without 12-hydroxylation. It was also found that the retention of taurine conjugates on the BEH C18 column was sensitive to the strength of formic acid and ammonium in mobile phases. By using the volatile buffers with an equivalent ammonium level as mobile phases, we comprehensively demonstrated the effects of the elution pH value on the retention behaviors of BAs on both the BEH C18 column and HSS T3 column. Based on the retention data acquired on a C18 column, we presented the ionization constants (pK a) of various BAs with the widest coverage beyond those of previous reports. When we made attempts to establish the structure-retention relationships (SRRs) of BAs, the lack of discriminative structural descriptors for BA stereoisomers emerged as the bottleneck problem. The methods and results presented in this work are especially useful for the development of reliable, sensitive, high-throughput, and robust LC-MS bioanalytical protocols for the quantitative metabolomic studies. Graphical Abstract Nonlinear curve fitting of capacity factors and elution pH value for the separation of common unconjugated bile acids.
Keywords: Bile acid; Electrospray ionization; High-performance liquid chromatography; Ionization constant; Mass spectrometry; Structure-retention relationship.
Figures

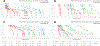

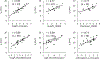
References
-
- Carey MC, Small DM (1972) Micelle formation by bile salts. Physical-chemical and thermodynamic considerations. Archives of internal medicine 130 (4):506–527 - PubMed
-
- Hofmann AF (1999) The continuing importance of bile acids in liver and intestinal disease. Archives of internal medicine 159 (22):2647–2658 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

