Expression Optimization of Anti-CD22 scFv-Apoptin Fusion Protein Using Experimental Design Methodology
- PMID: 28689385
- PMCID: PMC5712387
- DOI: 10.22034/ibj.22.1.66
Expression Optimization of Anti-CD22 scFv-Apoptin Fusion Protein Using Experimental Design Methodology
Abstract
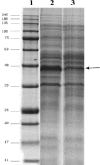
Background: Design of experiments is a rapid and cost-effective approach for optimization of recombinant protein production process. In our previous study, we generated a potent dual-acting fusion protein, anti-CD22 scFv-apoptin, to target B-cell malignant cell lines. In the present investigation, we report the effect of different variables on the expression levels of this fusion protein.
Methods: Four variables (cell optical density at induction, IPTG concentration, induction temperature, and induction time) were tested using experimental design.
Results: Our findings demonstrated that among the examined variables, only the induction time had a significant positive effect on the protein expression yield.
Conclusion: Experimental design was successfully applied in this study. The optimized condition obtained in the current study can be applied in future commercial production of this novel fusion protein.
Keywords: Recombinant protein; Single-chain antibodies; Fusion proteins; E. coli.
Conflict of interest statement
Figures
Similar articles
-
A novel anti-CD22 scFv-apoptin fusion protein induces apoptosis in malignant B-cells.AMB Express. 2017 Dec;7(1):112. doi: 10.1186/s13568-017-0410-5. Epub 2017 Jun 2. AMB Express. 2017. PMID: 28582973 Free PMC article.
-
High efficient expression of a functional humanized single-chain variable fragment (scFv) antibody against CD22 in Pichia pastoris.Appl Microbiol Biotechnol. 2014 Dec;98(24):10023-39. doi: 10.1007/s00253-014-6071-2. Epub 2014 Sep 21. Appl Microbiol Biotechnol. 2014. PMID: 25239038
-
A Novel Anti-CD22 scFv.Bim Fusion Protein Effectively Induces Apoptosis in Malignant B cells and Promotes Cytotoxicity.Appl Biochem Biotechnol. 2022 Dec;194(12):5878-5906. doi: 10.1007/s12010-022-04035-y. Epub 2022 Jul 15. Appl Biochem Biotechnol. 2022. PMID: 35838885
-
Implementation of a Design of Experiments to Improve Periplasmic Yield of Functional ScFv Antibodies in a Phage Display Platform.Adv Pharm Bull. 2022 May;12(3):583-592. doi: 10.34172/apb.2022.061. Epub 2021 Jul 3. Adv Pharm Bull. 2022. PMID: 35935041 Free PMC article.
-
Statistical optimization of culture conditions for expression of recombinant humanized anti-EpCAM single-chain antibody using response surface methodology.Res Pharm Sci. 2021 Mar 5;16(2):153-164. doi: 10.4103/1735-5362.310522. eCollection 2021 Apr. Res Pharm Sci. 2021. PMID: 34084202 Free PMC article.
Cited by
-
The mosaic puzzle of the therapeutic monoclonal antibodies and antibody fragments - A modular transition from full-length immunoglobulins to antibody mimetics.Leuk Res Rep. 2022 Jun 27;18:100335. doi: 10.1016/j.lrr.2022.100335. eCollection 2022. Leuk Res Rep. 2022. PMID: 35832747 Free PMC article.
-
Anti-HER2 scFv Expression in Escherichia coli SHuffle®T7 Express Cells: Effects on Solubility and Biological Activity.Mol Biotechnol. 2020 Jan;62(1):18-30. doi: 10.1007/s12033-019-00221-2. Mol Biotechnol. 2020. PMID: 31691197
References
-
- Wells E, Robinson AS. Cellular engineering for therapeutic protein production:product quality, host modification, and process improvement. Biotechnology journal. 2017;12(1) doi:10.1002/biot.201600105. - PubMed
-
- Gupta SK, Shukla P. Microbial platform technology for recombinant antibody fragment production:A review. Critical reviews in microbiology. 2017;43(1):31–42. - PubMed
-
- Gupta SK, Shukla P. Advanced technologies for improved expression of recombinant proteins in bacteria:perspectives and applications. Critical reviews in biotechnology. 2016;36(6):1089–1098. - PubMed
LinkOut - more resources
Full Text Sources
