Expression Optimization of Anti-CD22 scFv-Apoptin Fusion Protein Using Experimental Design Methodology
- PMID: 28689385
- PMCID: PMC5712387
- DOI: 10.22034/ibj.22.1.66
Expression Optimization of Anti-CD22 scFv-Apoptin Fusion Protein Using Experimental Design Methodology
Abstract
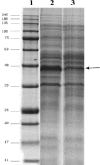
Background: Design of experiments is a rapid and cost-effective approach for optimization of recombinant protein production process. In our previous study, we generated a potent dual-acting fusion protein, anti-CD22 scFv-apoptin, to target B-cell malignant cell lines. In the present investigation, we report the effect of different variables on the expression levels of this fusion protein.
Methods: Four variables (cell optical density at induction, IPTG concentration, induction temperature, and induction time) were tested using experimental design.
Results: Our findings demonstrated that among the examined variables, only the induction time had a significant positive effect on the protein expression yield.
Conclusion: Experimental design was successfully applied in this study. The optimized condition obtained in the current study can be applied in future commercial production of this novel fusion protein.
Keywords: Recombinant protein; Single-chain antibodies; Fusion proteins; E. coli.
Conflict of interest statement
Figures
References
-
- Wells E, Robinson AS. Cellular engineering for therapeutic protein production:product quality, host modification, and process improvement. Biotechnology journal. 2017;12(1) doi:10.1002/biot.201600105. - PubMed
-
- Gupta SK, Shukla P. Microbial platform technology for recombinant antibody fragment production:A review. Critical reviews in microbiology. 2017;43(1):31–42. - PubMed
-
- Gupta SK, Shukla P. Advanced technologies for improved expression of recombinant proteins in bacteria:perspectives and applications. Critical reviews in biotechnology. 2016;36(6):1089–1098. - PubMed
LinkOut - more resources
Full Text Sources
