Severe Asthma Phenotypes - How Should They Guide Evaluation and Treatment?
- PMID: 28689840
- PMCID: PMC5541906
- DOI: 10.1016/j.jaip.2017.05.015
Severe Asthma Phenotypes - How Should They Guide Evaluation and Treatment?
Abstract
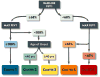
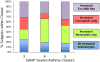
Although patients with "severe" asthma tend to be characterized by ongoing symptoms and airway inflammation despite treatment with high doses of inhaled and systemic corticosteroids, there is increasing recognition of marked phenotypic heterogeneity within affected patients. Although "precision medicine" approaches for patients with severe asthma are needed, there are many hurdles that must be overcome in daily practice. The National Heart, Lung and Blood Institute's Severe Asthma Research Program (SARP) has been at the forefront of phenotype discovery in severe asthma for the past decade. SARP, along with other international groups, has described clinical severe asthma phenotypes in both adults and children that can be evaluated in the clinical setting. Although these clinical phenotypes provide a good "starting point" for addressing disease heterogeneity in severe asthma in everyday practice, more efforts are needed to understand how these phenotypes relate to underlying disease mechanisms and pharmacological treatment responses. This review highlights the clinical asthma phenotypes identified to date, their associations with underlying endotypes and potential biomarkers, and remaining knowledge gaps that must be addressed before precision medicine can become a reality for patients with severe asthma.
Keywords: Biomarker; Cluster analysis; Endotype; Precision medicine; SARP; Severe asthma phenotypes.
Copyright © 2017 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Ward BW, Clarke TC, Freeman G, Schiller JS. Early release of selected estimates based on data from the January-June 2016 National Health Interview Survey. Centers for Disease Control and Prevention, National Center for Health Statistics. 2016 Nov;
-
- Slejko JF, Ghushchyan VH, Sucher B, Globe DR, Lin SL, Globe G, et al. Asthma control in the United States, 2008-2010: indicators of poor asthma control. J Allergy Clin Immunol. 2014;133:1579–87. - PubMed
-
- Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. - PubMed
-
- Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat. 2012;3:1–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

