The Role of Reactive Oxygen Species and Autophagy in Periodontitis and Their Potential Linkage
- PMID: 28690552
- PMCID: PMC5481360
- DOI: 10.3389/fphys.2017.00439
The Role of Reactive Oxygen Species and Autophagy in Periodontitis and Their Potential Linkage
Abstract
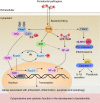
Periodontitis is a chronic inflammatory disease that causes damage to periodontal tissues, which include the gingiva, periodontal ligament, and alveolar bone. The major cause of periodontal tissue destruction is an inappropriate host response to microorganisms and their products. Specifically, a homeostatic imbalance between reactive oxygen species (ROS) and antioxidant defense systems has been implicated in the pathogenesis of periodontitis. Elevated levels of ROS acting as intracellular signal transducers result in autophagy, which plays a dual role in periodontitis by promoting cell death or blocking apoptosis in infected cells. Autophagy can also regulate ROS generation and scavenging. Investigations are ongoing to elucidate the crosstalk mechanisms between ROS and autophagy. Here, we review the physiological and pathological roles of ROS and autophagy in periodontal tissues. The redox-sensitive pathways related to autophagy, such as mTORC1, Beclin 1, and the Atg12-Atg5 complex, are explored in depth to provide a comprehensive overview of the crosstalk between ROS and autophagy. Based on the current evidence, we suggest that a potential linkage between ROS and autophagy is involved in the pathogenesis of periodontitis.
Keywords: Atg12-Atg5 complex; Beclin 1; JNK; NF-κB; autophagy; mTORC1; periodontitis; reactive oxygen species.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

