Protection of Mice from Acute Graft-versus-Host Disease Requires CD28 Co-stimulation on Donor CD4+ Foxp3+ Regulatory T Cells
- PMID: 28690612
- PMCID: PMC5481316
- DOI: 10.3389/fimmu.2017.00721
Protection of Mice from Acute Graft-versus-Host Disease Requires CD28 Co-stimulation on Donor CD4+ Foxp3+ Regulatory T Cells
Abstract
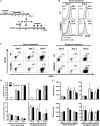
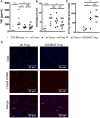
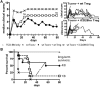
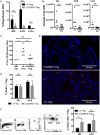
Acute graft-versus-host disease (aGvHD) is a major cause of morbidity and mortality after allogeneic hematopoietic stem cell plus T cell transplantation (allo-HSCT). In this study, we investigated the requirement for CD28 co-stimulation of donor CD4+ conventional (CD4+CD25-Foxp3-, Tconv) and regulatory (CD4+CD25+Foxp3+, Treg) T cells in aGvHD using tamoxifen-inducible CD28 knockout (iCD28KO) or wild-type (wt) littermates as donors of CD4+ Tconv and Treg. In the highly inflammatory C57BL/6 into BALB/c allo-HSCT transplantation model, CD28 depletion on donor CD4+ Tconv reduced clinical signs of aGvHD, but did not significantly prolong survival of the recipient mice. Selective depletion of CD28 on donor Treg did not abrogate protection of recipient mice from aGvHD until about day 20 after allo-HSCT. Later, however, the pool of CD28-depleted Treg drastically declined as compared to wt Treg. Consequently, only wt, but not CD28-deficient, Treg were able to continuously suppress aGvHD and induce long-term survival of the recipient mice. To our knowledge, this is the first study that specifically evaluates the impact of CD28 expression on donor Treg in aGvHD. Moreover, the delayed kinetics of aGvHD lethality after transplantation of iCD28KO Treg provides a novel animal model for similar disease courses found in patients after allo-HSCT.
Keywords: CD28; acute graft-versus-host disease; co-stimulation; inducible deletion; regulatory T cells.
Figures



Similar articles
-
[The role of CD4+ CD25+ T cell and FOXP3 in hsot acute graft rejection].Zhonghua Nei Ke Za Zhi. 2006 Oct;45(10):835-8. Zhonghua Nei Ke Za Zhi. 2006. PMID: 17217750 Chinese.
-
[Prophylactic effect of TLR5 agonist flagellin on acute graft versus host disease after allogeneic hematopoietic stem cell transplantation and its mechanism].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012 Aug;20(4):965-70. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2012. PMID: 22931665 Chinese.
-
ADAR1 improved Treg cell function through the miR-21b/Foxp3 axis and inhibits the progression of acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation.Int Immunopharmacol. 2023 Feb;115:109620. doi: 10.1016/j.intimp.2022.109620. Epub 2022 Dec 26. Int Immunopharmacol. 2023. PMID: 36577155
-
HIF-1α inhibitor echinomycin reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect.J Transl Med. 2017 Feb 10;15(1):28. doi: 10.1186/s12967-017-1132-9. J Transl Med. 2017. PMID: 28183349 Free PMC article.
-
CD28 co-stimulation in T-cell homeostasis: a recent perspective.Immunotargets Ther. 2015 May 28;4:111-22. doi: 10.2147/ITT.S61647. eCollection 2015. Immunotargets Ther. 2015. PMID: 27471717 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

