Novel Therapeutic Effects of Leonurine On Ischemic Stroke: New Mechanisms of BBB Integrity
- PMID: 28690765
- PMCID: PMC5485366
- DOI: 10.1155/2017/7150376
Novel Therapeutic Effects of Leonurine On Ischemic Stroke: New Mechanisms of BBB Integrity
Abstract
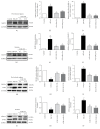
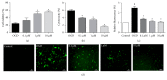
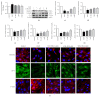
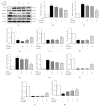
Stroke is a leading cause of morbidity and mortality globally. Leonurine (also named SCM-198), a compound extracted from Herba leonuri, was effective on the prevention of various cardiovascular and brain diseases. The purpose of this study was to explore the possible therapeutic potential of SCM-198 against ischemia reperfusion injury and underlying mechanisms. In the in vivo transient middle cerebral artery occlusion (tMCAO) rat model, we found that treatment with SCM-198 could decrease infarct volume and improve neurological deficit by protecting against blood-brain barrier (BBB) breakdown. In the in vitro model of cell oxygen-glucose deprivation and reoxygenation (OGD/R), consistent results were obtained with decreased reactive oxygen species (ROS) production and maintained the BBB integrity. Further study demonstrated that SCM-198 increased the expression of histone deacetylase- (HDAC-) 4 which could inhibit NADPH oxidase- (NOX-) 4 and matrix metalloproteinase- (MMP-) 9 expression, resulting in the elevation of tight junction proteins, including claudin-5, occludin, and zonula occluden- (ZO-) 1. These results indicated SCM-198 protected BBB integrity by regulating the HDAC4/NOX4/MMP-9 tight junction pathway. Our findings provided novel insights into the protective effects and mechanisms of SCM-198 on ischemic stroke, indicating SCM-198 as a new class of potential drug against acute onset of ischemic stroke.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

