Phagocytosis: A Fundamental Process in Immunity
- PMID: 28691037
- PMCID: PMC5485277
- DOI: 10.1155/2017/9042851
Phagocytosis: A Fundamental Process in Immunity
Abstract
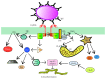
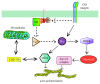
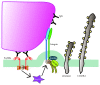
One hundred years have passed since the death of Élie Metchnikoff (1845-1916). He was the first to observe the uptake of particles by cells and realized the importance of this process for the host response to injury and infection. He also was a strong advocate of the role of phagocytosis in cellular immunity, and with this he gave us the basis for our modern understanding of inflammation and the innate and acquired immune responses. Phagocytosis is an elegant but complex process for the ingestion and elimination of pathogens, but it is also important for the elimination of apoptotic cells and hence fundamental for tissue homeostasis. Phagocytosis can be divided into four main steps: (i) recognition of the target particle, (ii) signaling to activate the internalization machinery, (iii) phagosome formation, and (iv) phagolysosome maturation. In recent years, the use of new tools of molecular biology and microscopy has provided new insights into the cellular mechanisms of phagocytosis. In this review, we present a general view of our current knowledge on phagocytosis. We emphasize novel molecular findings, particularly on phagosome formation and maturation, and discuss aspects that remain incompletely understood.
Figures

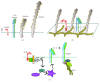

References
-
- Vikhanski L. Immunity: How Elie Metchnikoff changed the course of modern medicine. Chicago Review Press. 2016 doi: 10.1007/s12062-016-9166-y. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

