Covalent Organic Frameworks as a Platform for Multidimensional Polymerization
- PMID: 28691064
- PMCID: PMC5492257
- DOI: 10.1021/acscentsci.7b00127
Covalent Organic Frameworks as a Platform for Multidimensional Polymerization
Abstract
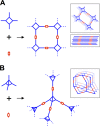
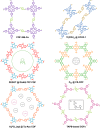
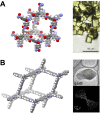
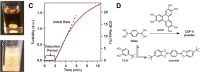
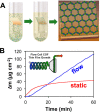
The simultaneous polymerization and crystallization of monomers featuring directional bonding designs provides covalent organic frameworks (COFs), which are periodic polymer networks with robust covalent bonds arranged in two- or three-dimensional topologies. The range of properties characterized in COFs has rapidly expanded to include those of interest for heterogeneous catalysis, energy storage and photovoltaic devices, and proton-conducting membranes. Yet many of these applications will require materials quality, morphological control, and synthetic efficiency exceeding the capabilities of contemporary synthetic methods. This level of control will emerge from an improved fundamental understanding of COF nucleation and growth processes. More powerful characterization of structure and defects, improved syntheses guided by mechanistic understanding, and accessing diverse isolated forms, ranging from single crystals to thin films to colloidal suspensions, remain important frontier problems.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Stupp S. I.; Palmer L. C. Supramolecular Chemistry and Self-Assembly in Organic Materials Design. Chem. Mater. 2014, 26, 507–518. 10.1021/cm403028b. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

