Record Atmospheric Fresh Water Capture and Heat Transfer with a Material Operating at the Water Uptake Reversibility Limit
- PMID: 28691080
- PMCID: PMC5492259
- DOI: 10.1021/acscentsci.7b00186
Record Atmospheric Fresh Water Capture and Heat Transfer with a Material Operating at the Water Uptake Reversibility Limit
Abstract
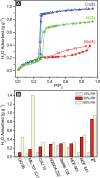
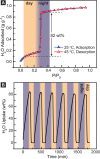
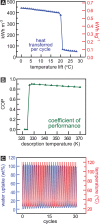
The capture of water vapor at low relative humidity is desirable for producing potable water in desert regions and for heat transfer and storage. Here, we report a mesoporous metal-organic framework that captures 82% water by weight below 30% relative humidity. Under simulated desert conditions, the sorbent would deliver 0.82 gH2O gMOF-1, nearly double the quantity of fresh water compared to the previous best material. The material further demonstrates a cooling capacity of 400 kWh m-3 per cycle, also a record value for a sorbent capable of creating a 20 °C difference between ambient and output temperature. The water uptake in this sorbent is optimized: the pore diameter of our material is above the critical diameter for water capillary action, enabling water uptake at the limit of reversibility.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.D., A.J.R., and MIT have filed a patent application pertaining to the results and materials presented here.
Figures

References
-
- United Nations World Water Assessment Programme.. UN World Water Development Report: Managing Water under Uncertainty and Risk, 4th ed.; 2012. Vol. 1, pp 1–407.
-
- 2030 Water Resources Group. Charting Our Water Future; 2009.
LinkOut - more resources
Full Text Sources
Other Literature Sources

