Peptide backbone circularization enhances antifreeze protein thermostability
- PMID: 28691252
- PMCID: PMC5606537
- DOI: 10.1002/pro.3228
Peptide backbone circularization enhances antifreeze protein thermostability
Abstract
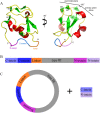
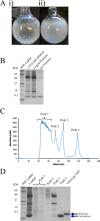
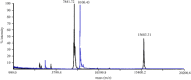
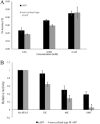
Antifreeze proteins (AFPs) are a class of ice-binding proteins that promote survival of a variety of cold-adapted organisms by decreasing the freezing temperature of bodily fluids. A growing number of biomedical, agricultural, and commercial products, such as organs, foods, and industrial fluids, have benefited from the ability of AFPs to control ice crystal growth and prevent ice recrystallization at subzero temperatures. One limitation of AFP use in these latter contexts is their tendency to denature and irreversibly lose activity at the elevated temperatures of certain industrial processing or large-scale AFP production. Using the small, thermolabile type III AFP as a model system, we demonstrate that AFP thermostability is dramatically enhanced via split intein-mediated N- and C-terminal end ligation. To engineer this circular protein, computational modeling and molecular dynamics simulations were applied to identify an extein sequence that would fill the 20-Å gap separating the free ends of the AFP, yet impose little impact on the structure and entropic properties of its ice-binding surface. The top candidate was then expressed in bacteria, and the circularized protein was isolated from the intein domains by ice-affinity purification. This circularized AFP induced bipyramidal ice crystals during ice growth in the hysteresis gap and retained 40% of this activity even after incubation at 100°C for 30 min. NMR analysis implicated enhanced thermostability or refolding capacity of this protein compared to the noncyclized wild-type AFP. These studies support protein backbone circularization as a means to expand the thermostability and practical applications of AFPs.
Keywords: antifreeze protein; backbone circularization; freezing hysteresis; protein stability; split intein; thermal stability.
© 2017 The Protein Society.
Figures


References
-
- Davies PL (2014) Ice‐binding proteins: a remarkable diversity of structures for stopping and starting ice growth. Trends Biochem Sci 39:548–555. - PubMed
-
- Regand A, Goff HD (2006) Ice recrystallization inhibition in ice cream as affected by ice structuring proteins from winter wheat grass. J Dairy Sci 89:49–57. - PubMed
-
- Zhang C, Zhang H, Wang L, Gao H, Guo XN, Yao HY (2007) Improvement of texture properties and flavor of frozen dough by carrot (Daucus carota) antifreeze protein supplementation. J Agric Food Chem 55:9620–9626. - PubMed
-
- Yeh CM, Kao BY, Peng HJ (2009) Production of a recombinant type 1 antifreeze protein analogue by L. lactis and its applications on frozen meat and frozen dough. J Agric Food Chem 57:6216–6223. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

