Synthetic lethality between the cohesin subunits STAG1 and STAG2 in diverse cancer contexts
- PMID: 28691904
- PMCID: PMC5531830
- DOI: 10.7554/eLife.26980
Synthetic lethality between the cohesin subunits STAG1 and STAG2 in diverse cancer contexts
Abstract
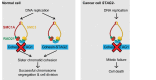
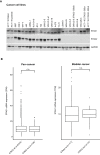
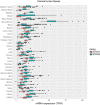
Recent genome analyses have identified recurrent mutations in the cohesin complex in a wide range of human cancers. Here we demonstrate that the most frequently mutated subunit of the cohesin complex, STAG2, displays a strong synthetic lethal interaction with its paralog STAG1. Mechanistically, STAG1 loss abrogates sister chromatid cohesion in STAG2 mutated but not in wild-type cells leading to mitotic catastrophe, defective cell division and apoptosis. STAG1 inactivation inhibits the proliferation of STAG2 mutated but not wild-type bladder cancer and Ewing sarcoma cell lines. Restoration of STAG2 expression in a mutated bladder cancer model alleviates the dependency on STAG1. Thus, STAG1 and STAG2 support sister chromatid cohesion to redundantly ensure cell survival. STAG1 represents a vulnerability of cancer cells carrying mutations in the major emerging tumor suppressor STAG2 across different cancer contexts. Exploiting synthetic lethal interactions to target recurrent cohesin mutations in cancer, e.g. by inhibiting STAG1, holds the promise for the development of selective therapeutics.
Keywords: cancer biology; cell biology; cell division; chromosomes; cohesin; genetic interaction; human; mitosis; synthetic lethality.
Conflict of interest statement
SL: Simone Lieb is a full-time employee of Boehringer Ingelheim RCV.
AS: Andreas Schlattl is a full-time employee of Boehringer Ingelheim RCV.
MAP: Mark Pearson is a full-time employee of Boehringer Ingelheim RCV.
NK: Norbert Kraut is a full-time employee of Boehringer Ingelheim RCV.
MP: Mark Petronczki is a full-time employee of Boehringer Ingelheim RCV.
The other authors declare that no competing interests exist.
Figures
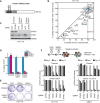






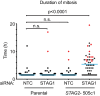

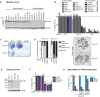
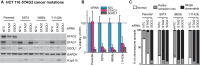

References
-
- Balbás-Martínez C, Sagrera A, Carrillo-de-Santa-Pau E, Earl J, Márquez M, Vazquez M, Lapi E, Castro-Giner F, Beltran S, Bayés M, Carrato A, Cigudosa JC, Domínguez O, Gut M, Herranz J, Juanpere N, Kogevinas M, Langa X, López-Knowles E, Lorente JA, Lloreta J, Pisano DG, Richart L, Rico D, Salgado RN, Tardón A, Chanock S, Heath S, Valencia A, Losada A, Gut I, Malats N, Real FX. Recurrent inactivation of STAG2 in bladder Cancer is not associated with aneuploidy. Nature Genetics. 2013;45:1464–1469. doi: 10.1038/ng.2799. - DOI - PMC - PubMed
-
- Ban J, Aryee DN, Fourtouna A, van der Ent W, Kauer M, Niedan S, Machado I, Rodriguez-Galindo C, Tirado OM, Schwentner R, Picci P, Flanagan AM, Berg V, Strauss SJ, Scotlandi K, Lawlor ER, Snaar-Jagalska E, Llombart-Bosch A, Kovar H. Suppression of deacetylase SIRT1 mediates tumor-suppressive NOTCH response and offers a novel treatment option in metastatic Ewing sarcoma. Cancer Research. 2014;74:6578–6588. doi: 10.1158/0008-5472.CAN-14-1736. - DOI - PubMed
-
- Barber TD, McManus K, Yuen KW, Reis M, Parmigiani G, Shen D, Barrett I, Nouhi Y, Spencer F, Markowitz S, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C, Hieter P. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. PNAS. 2008;105:3443–3448. doi: 10.1073/pnas.0712384105. - DOI - PMC - PubMed
-
- Brohl AS, Solomon DA, Chang W, Wang J, Song Y, Sindiri S, Patidar R, Hurd L, Chen L, Shern JF, Liao H, Wen X, Gerard J, Kim JS, Lopez Guerrero JA, Machado I, Wai DH, Picci P, Triche T, Horvai AE, Miettinen M, Wei JS, Catchpool D, Llombart-Bosch A, Waldman T, Khan J. The genomic landscape of the Ewing Sarcoma family of tumors reveals recurrent STAG2 mutation. PLoS Genetics. 2014;10:e1004475. doi: 10.1371/journal.pgen.1004475. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

