Using biomaterials to rewire the process of wound repair
- PMID: 28692083
- PMCID: PMC5576529
- DOI: 10.1039/c7bm00295e
Using biomaterials to rewire the process of wound repair
Abstract
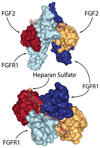
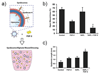
Wound healing is one of the most complex processes that our bodies must perform. While our ability to repair wounds is often taken for granted, conditions such as diabetes, obesity, or simply old age can significantly impair this process. With the incidence of all three predicted to continue growing into the foreseeable future, there is an increasing push to develop strategies that facilitate healing. Biomaterials are an attractive approach for modulating all aspects of repair, and have the potential to steer the healing process towards regeneration. In this review, we will cover recent advances in developing biomaterials that actively modulate the process of wound healing, and will provide insight into how biomaterials can be used to simultaneously rewire multiple phases of the repair process.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

