A discontinuous autoinhibitory module masks the A1 domain of von Willebrand factor
- PMID: 28692141
- PMCID: PMC5585049
- DOI: 10.1111/jth.13775
A discontinuous autoinhibitory module masks the A1 domain of von Willebrand factor
Abstract
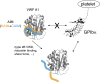
Essentials The mechanism for the auto-inhibition of von Willebrand factor (VWF) remains unclear. Hydrogen exchange of two VWF A1 fragments with disparate activities was measured and compared. Discontinuous residues flanking A1 form a structural module that blocks A1 binding to the platelet. Our results suggest a potentially unified model of VWF activation. Click to hear an ISTH Academy presentation on the domain architecture of VWF and activation by elongational flow by Dr Springer SUMMARY: Background How von Willebrand factor (VWF) senses and responds to shear flow remains unclear. In the absence of shear flow, VWF or its fragments can be induced to bind spontaneously to platelet GPIbα. Objectives To elucidate the auto-inhibition mechanism of VWF. Methods Hydrogen-deuterium exchange (HDX) of two recombinant VWF fragments expressed from baby hamster kidney cells were measured and compared. Results The shortA1 protein contains VWF residues 1261-1472 and binds GPIbα with a significantly higher affinity than the longA1 protein that contains VWF residues 1238-1472. Both proteins contain the VWF A1 domain (residues 1272-1458). Many residues in longA1, particularly those in the N- and C-terminal sequences flanking the A1 domain, and in helix α1, loops α1β2 and β3α2, demonstrated markedly reduced HDX compared with their counterparts in shortA1. The HDX-protected region in longA1 overlaps with the GPIbα-binding interface and is clustered with type 2B von Willebrand disease (VWD) mutations. Additional comparison with the HDX of denatured longA1 and ristocetin-bound longA1 indicates the N- and C-terminal sequences flanking the A1 domain form cooperatively an integrated autoinhibitory module (AIM) that interacts with the HDX-protected region. Binding of ristocetin to the C-terminal part of the AIM desorbs the AIM from A1 and enables longA1 binding to GPIbα. Conclusion The discontinuous AIM binds the A1 domain and prevents it from binding to GPIbα, which has significant implications for the pathogenesis of type 2B VWD and the shear-induced activation of VWF activity.
Keywords: Type 2B von Willebrand disease; blood platelet; ristocetin; tandem mass spectrometry; von Willebrand factor.
© 2017 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures



References
-
- Wagner DD. Cell biology of von Willebrand factor. Annu Rev Cell Biol. 1990;6:217–246. - PubMed
-
- Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. - PubMed
-
- Slayter H, Loscalzo J, Bockenstedt P, Handin RI. Native conformation of human von Willebrand protein. Analysis by electron microscopy and quasi-elastic light scattering. J. Biol.Chem. 1985;260:8559–8563. - PubMed
-
- Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. - PubMed
-
- Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, Marchant RE. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88:2939–2950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

