Poor efficacy of preemptive amoxicillin clavulanate for preventing secondary infection from Bothrops snakebites in the Brazilian Amazon: A randomized controlled clinical trial
- PMID: 28692641
- PMCID: PMC5519217
- DOI: 10.1371/journal.pntd.0005745
Poor efficacy of preemptive amoxicillin clavulanate for preventing secondary infection from Bothrops snakebites in the Brazilian Amazon: A randomized controlled clinical trial
Abstract
Background: Secondary bacterial infections from snakebites contribute to the high complication rates that can lead to permanent function loss and disabilities. Although common in endemic areas, routine empirical prophylactic use of antibiotics aiming to prevent secondary infection lacks a clearly defined policy. The aim of this work was to estimate the efficacy of amoxicillin clavulanate for reducing the secondary infection incidence in patients bitten by Bothrops snakes, and, secondarily, identify risk factors for secondary infections from snakebites in the Western Brazilian Amazon.
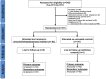
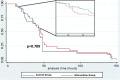
Methods and findings: This was an open-label, two-arm individually randomized superiority trial to prevent secondary infection from Bothrops snakebites. The antibiotic chosen for this clinical trial was oral amoxicillin clavulanate per seven days compared to no intervention. A total of 345 patients were assessed for eligibility in the study period. From this total, 187 accomplished the inclusion criteria and were randomized, 93 in the interventional group and 94 in the untreated control group. All randomized participants completed the 7 days follow-up period. Enzyme immunoassay confirmed Bothrops envenoming diagnosis in all participants. Primary outcome was defined as secondary infection (abscess and/or cellulitis) until day 7 after admission. Secondary infection incidence until 7 days after admission was 35.5% in the intervention group and 44.1% in the control group [RR = 0.80 (95%CI = 0.56 to 1.15; p = 0.235)]. Survival analysis demonstrated that the time from patient admission to the onset of secondary infection was not different between amoxicillin clavulanate treated and control group (Log-rank = 2.23; p = 0.789).Secondary infections incidence in 7 days of follow-up was independently associated to fibrinogen >400 mg/dL [AOR = 4.78 (95%CI = 2.17 to 10.55; p<0.001)], alanine transaminase >44 IU/L [AOR = 2.52 (95%CI = 1.06 to 5.98; p = 0.037)], C-reactive protein >6.5 mg/L [AOR = 2.98 (95%CI = 1.40 to 6.35; p = 0.005)], moderate pain [AOR = 24.30 (95%CI = 4.69 to 125.84; p<0.001)] and moderate snakebites [AOR = 2.43 (95%CI = 1.07 to 5.50; p = 0.034)].
Conclusions/significance: Preemptive amoxicillin clavulanate was not effective for preventing secondary infections from Bothrops snakebites. Laboratorial markers, such as high fibrinogen, alanine transaminase and C-reactive protein levels, and severity clinical grading of snakebites, may help to accurately diagnose secondary infections.
Trial registration: Brazilian Clinical Trials Registry (ReBec): RBR-3h33wy; UTN Number: U1111-1169-1005.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- White J (2000) Bites and stings from venomous animals: a global overview. Ther Drug Monit 22: 65–8. - PubMed
-
- Gutiérrez JM, Theakston RDG, Warrell D (2006) Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PLoS Med 3: e150 doi: 10.1371/journal.pmed.0030150 - DOI - PMC - PubMed
-
- Kasturiratne A, Wickremasinghe AR, Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. (2008) The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med 5: 1591–1604. doi: 10.1371/journal.pmed.0050218 - DOI - PMC - PubMed
-
- Warrell DA (2010). Snake bite. Lancet 375: 77–88. doi: 10.1016/S0140-6736(09)61754-2 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

