Safety of Converting From Tetrabenazine to Deutetrabenazine for the Treatment of Chorea
- PMID: 28692723
- PMCID: PMC5710322
- DOI: 10.1001/jamaneurol.2017.1352
Safety of Converting From Tetrabenazine to Deutetrabenazine for the Treatment of Chorea
Erratum in
-
Incorrect Affiliation.JAMA Neurol. 2018 Jan 1;75(1):133. doi: 10.1001/jamaneurol.2017.3563. JAMA Neurol. 2018. PMID: 29131881 Free PMC article. No abstract available.
Abstract
Importance: Tetrabenazine is efficacious for chorea control; however, tolerability concerns exist. Deutetrabenazine, a novel molecule that reduces chorea, was well tolerated in a double-blind, placebo-controlled study.
Objectives: To evaluate the safety and explore the efficacy of conversion from tetrabenazine to deutetrabenazine in patients with chorea associated with Huntington disease (HD).
Design, setting, and participants: In this ongoing, open-label, single-arm study that started on December 21, 2013, 37 patients at 13 Huntington Study Group sites in the United States and Australia who were taking stable doses of tetrabenazine that provided a therapeutic benefit were switched overnight to deutetrabenazine therapy. After week 1, the deutetrabenazine dose was titrated on a weekly basis for optimal chorea control.
Interventions: Deutetrabenazine administration at a dosage thought to provide comparable systemic exposure to the active metabolites of the prior, stable tetrabenazine regimen.
Main outcomes and measures: Safety measures included adverse events (AEs), clinical laboratory tests, vital signs, electrocardiograms, and validated scales. Changes in the Unified Huntington's Disease Rating Scale total maximal chorea score and total motor score were efficacy end points.
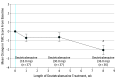
Results: Of the 53 patients with HD screened for the study, 37 ambulatory patients with manifest HD (mean [SD] age, 52.4 [11.5] years; 22 [59%] male and 15 [41%] female; 36 white [97.3%]) were enrolled. Deutetrabenazine was generally well tolerated, with low rates of neuropsychiatric AEs. Safety scales did not reveal subclinical toxicity with deutetrabenazine treatment. Rates of dose reduction or suspension attributable to AEs were also low. Chorea control, as measured by the total maximal chorea score, was maintained at week 1 and significantly improved at week 8 (mean [SD] change from baseline, 2.1 [3.2]; P < .001).
Conclusions and relevance: In patients with chorea, overnight conversion to deutetrabenazine therapy provided a favorable safety profile and effectively maintained chorea control.
Conflict of interest statement
Figures


References
-
- Jankovic J, Roos RA. Chorea associated with Huntington’s disease: to treat or not to treat? Mov Disord. 2014;29(11):1414-1418. - PubMed
-
- Kenney C, Hunter C, Jankovic J. Long-term tolerability of tetrabenazine in the treatment of hyperkinetic movement disorders. Mov Disord. 2007;22(2):193-197. - PubMed
-
- Jankovic J. Dopamine depleters in the treatment of hyperkinetic movement disorders. Expert Opin Pharmacother. 2016;17(18):2461-2470. - PubMed
-
- AustedoTM [package insert]. North Wales, PA: Teva Pharmaceuticals Inc; 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

