Effect of a Baby-Led Approach to Complementary Feeding on Infant Growth and Overweight: A Randomized Clinical Trial
- PMID: 28692728
- PMCID: PMC5710413
- DOI: 10.1001/jamapediatrics.2017.1284
Effect of a Baby-Led Approach to Complementary Feeding on Infant Growth and Overweight: A Randomized Clinical Trial
Abstract
Importance: Baby-led approaches to complementary feeding, which promote self-feeding of all nonliquid foods are proposed to improve energy self-regulation and lower obesity risk. However, to date, no randomized clinical trials have studied this proposition.
Objective: To determine whether a baby-led approach to complementary feeding results in a lower body mass index (BMI) than traditional spoon-feeding.
Design, setting, and participants: The 2-year Baby-Led Introduction to Solids (BLISS) randomized clinical trial recruited 206 women (168 [81.6%] of European ancestry; 85 [41.3%] primiparous) in late pregnancy from December 19, 2012, through March 17, 2014, as part of a community intervention in Dunedin, New Zealand. Women were randomized to a control condition (n = 101) or the BLISS intervention (n = 105) after stratification for parity and education. All outcomes were collected by staff blinded to group randomization, and no participants withdrew because of an adverse event. Data were analyzed based on intention to treat.
Interventions: Mothers in the BLISS group received lactation consultant support (≥5 contacts) to extend exclusive breastfeeding and delay introduction of complementary foods until 6 months of age and 3 personalized face-to-face contacts (at 5.5, 7.0, and 9.0 months).
Main outcomes and measures: The primary outcome was BMI z score (at 12 and 24 months). Secondary outcomes included energy self-regulation and eating behaviors assessed with questionnaires at 6, 12, and 24 months and energy intake assessed with 3-day weighed diet records at 7, 12, and 24 months.
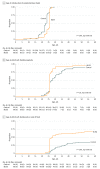
Results: Among the 206 participants (mean [SD] age, 31.3 [5.6] years), 166 were available for analysis at 24 months (retention, 80.5%). The mean (SD) BMI z score was not significantly different at 12 months (control group, 0.20 [0.89]; BLISS group, 0.44 [1.13]; adjusted difference, 0.21; 95% CI, -0.07 to 0.48) or at 24 months (control group, 0.24 [1.01]; BLISS group, 0.39 [1.04]; adjusted difference, 0.16; 95% CI, -0.13 to 0.45). At 24 months, 5 of 78 infants (6.4%) were overweight (BMI≥95th percentile) in the control group compared with 9 of 87 (10.3%) in the BLISS group (relative risk, 1.8; 95% CI, 0.6-5.7). Lower satiety responsiveness was observed in BLISS infants at 24 months (adjusted difference, -0.24; 95% CI, -0.41 to -0.07). Parents also reported less food fussiness (adjusted difference, -0.33; 95% CI, -0.51 to -0.14) and greater enjoyment of food (adjusted difference, 0.25; 95% CI, 0.07 to 0.43) at 12 months in BLISS infants. Estimated differences in energy intake were 55 kJ (95% CI, -284 to 395 kJ) at 12 months and 143 kJ (95% CI, -241 to 526 kJ) at 24 months.
Conclusions and relevance: A baby-led approach to complementary feeding did not result in more appropriate BMI than traditional spoon-feeding, although children were reported to have less food fussiness. Further research should determine whether these findings apply to individuals using unmodified baby-led weaning.
Trial registration: http://anzctr.org.au Identifier: ACTRN12612001133820.
Conflict of interest statement
Figures
Comment in
-
Baby-Led Weaning-Safe and Effective but Not Preventive of Obesity.JAMA Pediatr. 2017 Sep 1;171(9):832-833. doi: 10.1001/jamapediatrics.2017.1766. JAMA Pediatr. 2017. PMID: 28692709 No abstract available.
-
Baby-led weaning did not significantly impact body mass index when compared with traditional spoon-feeding.Arch Dis Child Educ Pract Ed. 2018 Aug;103(4):222. doi: 10.1136/archdischild-2017-314039. Epub 2017 Oct 9. Arch Dis Child Educ Pract Ed. 2018. PMID: 28993430 No abstract available.
-
A Baby-Led Approach to Complementary Feeding-Reply.JAMA Pediatr. 2018 Feb 1;172(2):197-198. doi: 10.1001/jamapediatrics.2017.4683. JAMA Pediatr. 2018. PMID: 29255851 No abstract available.
-
A Baby-Led Approach to Complementary Feeding.JAMA Pediatr. 2018 Feb 1;172(2):197. doi: 10.1001/jamapediatrics.2017.4680. JAMA Pediatr. 2018. PMID: 29255887 No abstract available.
References
-
- Ministry of Health A Portrait of Health: Key Results of the 20011/12 New Zealand Health Survey. Wellington, New Zealand: Ministry of Health; 2008.
-
- United States Department of Agriculture Infant feeding guide, chapter 5: complementary foods. 2009:101-128. http://wicworks.nal.usda.gov/infants/infant-feeding-guide. Modified May 26, 2017. Accessed November 2014.
-
- National Health and Medical Research Council (NHMRC) Infant Feeding Guidelines: Information for Health Workers. Canberra: Department of Health and Ageing, Australian Government; 2012.
-
- Rapley G, Murkett T. Baby-led Weaning. London, England: Vermilion; 2008.
-
- Rapley G. Baby-led weaning: transitioning to solid foods at the baby’s own pace. Community Pract. 2011;84(6):20-23. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

