Quantitative analysis and clonal characterization of T-cell receptor β repertoires in patients with advanced non-small cell lung cancer treated with cancer vaccine
- PMID: 28693166
- PMCID: PMC5494838
- DOI: 10.3892/ol.2017.6125
Quantitative analysis and clonal characterization of T-cell receptor β repertoires in patients with advanced non-small cell lung cancer treated with cancer vaccine
Abstract
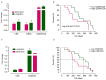
With the development of cancer immunotherapy that may activate T cells, a practical and quantitative method to improve monitoring and/or prediction of immunological response of patients as a predictive biomarker is of importance. To examine possible biomarkers for a therapeutic cancer vaccine containing a mixture of three epitope peptides derived from cell division-associated 1, lymphocyte antigen 6 complex locus K and insulin-like growth factor-II mRNA-binding protein 3, T-cell receptor β (TCRβ) repertoires of blood samples from 24 patients with human leukocyte antigen-A*2402-positive non-small cell lung cancer were characterized prior to and following 8 weeks of the cancer vaccine treatment, by applying a next-generation sequencing method. It was identified that 14 patients with overall survival (OS) times of ≥12 months had significantly lower TCRβ diversity indexes in samples prior to treatment, compared with 10 patients who succumbed within 1 year (P=0.03). In addition, patients with a high level of activated CD8+ T cells that are defined by a high granzyme A/CD8 ratio had favorable OS rates (log-rank test, P=0.04). The TCRβ diversity index and immunogenic gene markers following vaccine administration may serve as predictive or monitoring biomarkers for cancer vaccine treatment.
Keywords: T-cell receptor; cancer vaccine; diversity index; immunogenicity; lung cancer; next-generation sequencing; non-small cell lung cancer.
Figures

References
-
- Global status report on noncommunicable diseases 2014. World Health Organization; Geneva: 2014. pp. 11–14.
-
- Cancer Facts & Figures 2014. American Cancer Society; Atlanta, GA: 2014. pp. 14–15.
-
- Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
