Active children through individual vouchers - evaluation (ACTIVE): protocol for a mixed method randomised control trial to increase physical activity levels in teenagers
- PMID: 28693484
- PMCID: PMC5504609
- DOI: 10.1186/s12889-017-4554-7
Active children through individual vouchers - evaluation (ACTIVE): protocol for a mixed method randomised control trial to increase physical activity levels in teenagers
Erratum in
-
Erratum to: BMC Public Health, Vol. 18.BMC Public Health. 2017 Sep 22;17(1):736. doi: 10.1186/s12889-017-4709-6. BMC Public Health. 2017. PMID: 28938882 Free PMC article. No abstract available.
Abstract
Background: Many teenagers are insufficiently active despite the health benefits of physical activity (PA). There is strong evidence to show that inactivity and low fitness levels increase the risk of non-communicable diseases such as coronary heart disease (CHD), type 2 diabetes and breast and colon cancers (Lee et al. Lancet 380:219-29, 2012). A major barrier facing adolescents is accessibility (e.g. cost and lack of local facilities). The ACTIVE project aims to tackle this barrier through a multi-faceted intervention, giving teenagers vouchers to spend on activities of their choice and empowering young people to improve their fitness and PA levels.
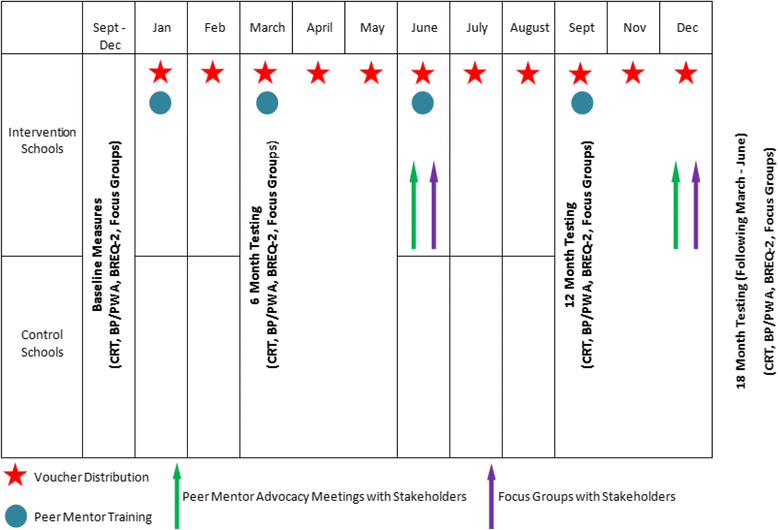
Design: ACTIVE is a mixed methods randomised control trial in 7 secondary schools in Swansea, South Wales. Quantitative and qualitative measures including PA (cooper run test (CRT), accelerometery over 7 days), cardiovascular (CV) measures (blood pressure, pulse wave analysis) and focus groups will be undertaken at 4 separate time points (baseline, 6 months,12 months and follow-up at 18 months). Intervention schools will receive a multi-component intervention involving 12 months of £20 vouchers to spend on physical activities of their choice, a peer mentor scheme and opportunities to attend advocacy meetings. Control schools are encouraged to continue usual practice. The primary aim is to examine the effect of the intervention in improving cardiovascular fitness.
Discussion: This paper describes the protocol for the ACTIVE randomised control trial, which aims to increase fitness, physical activity and socialisation of teenagers in Swansea, UK via a voucher scheme combined with peer mentoring. Results can contribute to the evidence base on teenage physical activity and, if effective, the intervention has the potential to inform future physical activity interventions and policy.
Trial registration: ISRCTN75594310 (Assigned 06/03/2017).
Keywords: Fitness; Mixed methods; Peer mentor; Physical activity; Teenagers; Voucher.
Conflict of interest statement
Ethics approval and consent to participate
The College of Human and Health Science Ethics Committee at the College of Medicine, Swansea University granted ACTIVE ethical approval on 12/05/2016 (Reference: 090516). Participant consent for primary and secondary outcomes was voluntary and involved parental consent and pupil assent forms.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Figures
References
-
- Lawlor DA, Jago R, Noble SM, Chittleborough CR, Campbell R, Mytton J, et al. The Active for Life Year 5 (AFLY5) school based cluster randomised controlled trial: Study protocol for a randomized controlled trial. Trials. 2011;12:no pagination-no pagination. doi:10.1186/1745-6215-12-181. - PMC - PubMed
-
- Bradley RH, Houts RM, Mcritchie SL, Brien MO. From ages 9 to 15 years. Child Aust. 2008;300:295–305. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials