Strengthening primary health care teams with palliative care leaders: protocol for a cluster randomized clinical trial
- PMID: 28693520
- PMCID: PMC5504625
- DOI: 10.1186/s12904-017-0217-9
Strengthening primary health care teams with palliative care leaders: protocol for a cluster randomized clinical trial
Erratum in
-
Erratum to: BMC Palliative Care, Vol. 17.BMC Palliat Care. 2017 Oct 10;16(1):51. doi: 10.1186/s12904-017-0233-9. BMC Palliat Care. 2017. PMID: 29017489 Free PMC article. No abstract available.
Abstract
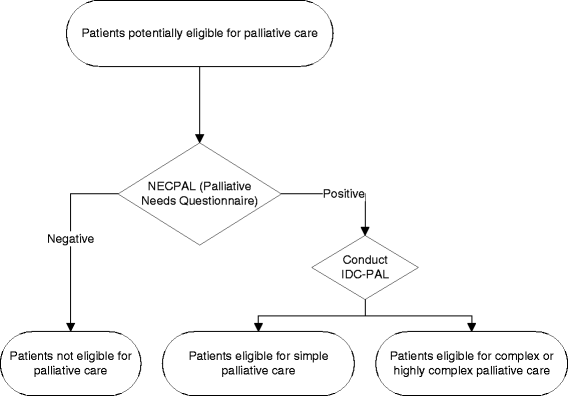
Background: The objective of the Balearic Islands Palliative Care (PC) Program is to improve the quality of PC through a shared model consisting of primary health care professionals, home-based PC teams, and PC units in hospitals. According to the World Health Organization (WHO), patients with advanced cancer and other terminal diseases benefit from early identification and proactive PC. We will evaluate the effectiveness of an intervention in which a PC leader is established in the primary health care center, and assess the effect of this intervention on the early identification of patients in need of PC, the efficient use of health care services, and direct health care costs.
Methods: Design: A two-arm cluster randomized clinical trial of 30 Primary Health Care Centers (PHCC) in Mallorca (Spain), in which each center was randomized to an intervention arm or a usual care arm. We expect that the number of patients identified as suitable for PC (including non-oncological PC) is at least 5% greater in the intervention arm.
Sample size: A total of 4640 deceased patients. Outcomes will be assessed by a blinded external review of the electronic records.
Interventions: General practitioners (GPs) and nurse leaders in PC for each PHCC will be appointed. These leaders will help promote PC training of colleagues, improve symptom management and psychological support of patients, and evaluate the complexity of individual cases so that these cases receive assistance from PC home-based teams.
Measurements: Early identification (>90 days before death), evaluation of case complexity, level of case complexity (with referral to a home-based PC team), use and cost of hospital and primary care services, and quality of life during the last month of life (≥2 emergency room visits, ≥2 hospital admissions, ≥14 days of hospitalization).
Discusion: PC leaders in primary care teams will improve the early identification of patients eligible for PC. This initiative could improve the quality of end-of-life care and utilization of hospital resources.
Trial registration: ISRCTN Registry identifier: ISRCTN92479122 . Retrospectively registered on 28 February 2017.
Keywords: End of life; Integrated care; Outcome assessment; Palliative care; Primary care; Program development; Public health care.
Conflict of interest statement
Authors’ information
Non applicable.
Ethics approval and consent to participate
This study will follow the principles outlined in the Declaration of Helsinki. Our study protocol has been approved by the Primary Care Research Committee, the Mallorca Ethical Committee of Clinical Research (IB 3170/16). Any protocol modification will be approved by the executive committee and submitted to the ethic committee for approval and noted to ISRCTN register. Exempt criteria for participant informed consent was applied since the research study involved the retrospective review, collection and analysis of medical record information and patients could not be identified. Trial participants will be notified if relevant protocol changes will be made.
Consent for publication
Non applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Hall S, Petkova H, Tsouros AD, et al. Palliative care for older people: better practices. Copenhagen: World Health Organization Regional Office for Europe; 2011.
-
- de España G. Estrategias en Cuidados Paliativos del Sistema Nacional de Salud [sede Web] Actualización 2010–2014. Madrid: Ministerio de Sanidad y Política Social e Igualdad; 2011.
-
- Doblado R, Herrera E, Librada S, Muñoz I, Rodriguez Z. Directorio de Recursos de Cuidados Paliativos en España. Monogr SECPAL. 2016;8
-
- Expert Committee. Cancer pain relief and palliative care. Geneva: World Health Organization; 1990. Technical Report Series. p. 804. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical