Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice
- PMID: 28693603
- PMCID: PMC5504676
- DOI: 10.1186/s13395-017-0132-z
Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice
Abstract
Background: Pax7+ satellite cells are required for skeletal muscle fiber growth during post-natal development in mice. Satellite cell-mediated myonuclear accretion also appears to persist into early adulthood. Given the important role of satellite cells during muscle development, we hypothesized that the necessity of satellite cells for adaptation to an imposed hypertrophic stimulus depends on maturational age.
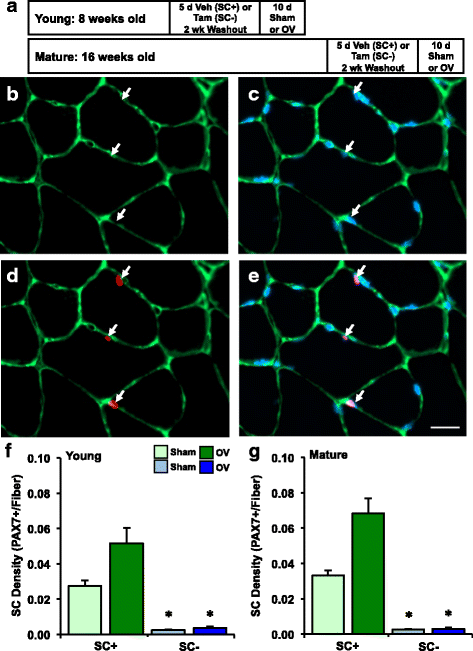
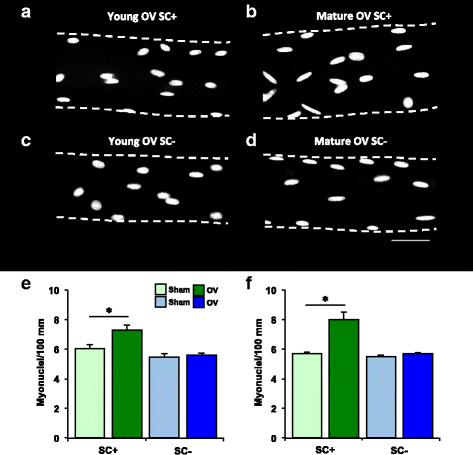
Methods: Pax7CreER-R26RDTA mice were treated for 5 days with vehicle (satellite cell-replete, SC+) or tamoxifen (satellite cell-depleted, SC-) at 2 months (young) and 4 months (mature) of age. Following a 2-week washout, mice were subjected to sham surgery or 10 day synergist ablation overload of the plantaris (n = 6-9 per group). The surgical approach minimized regeneration, de novo fiber formation, and fiber splitting while promoting muscle fiber growth. Satellite cell density (Pax7+ cells/fiber), embryonic myosin heavy chain expression (eMyHC), and muscle fiber cross sectional area (CSA) were evaluated via immunohistochemistry. Myonuclei (myonuclei/100 mm) were counted on isolated single muscle fibers.
Results: Tamoxifen treatment depleted satellite cells by ≥90% and prevented myonuclear accretion with overload in young and mature mice (p < 0.05). Satellite cells did not recover in SC- mice after overload. Average muscle fiber CSA increased ~20% in young SC+ (p = 0.07), mature SC+ (p < 0.05), and mature SC- mice (p < 0.05). In contrast, muscle fiber hypertrophy was prevented in young SC- mice. Muscle fiber number increased only in mature mice after overload (p < 0.05), and eMyHC expression was variable, specifically in mature SC+ mice.
Conclusions: Reliance on satellite cells for overload-induced hypertrophy is dependent on maturational age, and global responses to overload differ in young versus mature mice.
Keywords: Development; Fiber splitting; Muscle hypertrophy; Pax7; Regeneration; Synergist ablation.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Katz B. The terminations of the afferent nerve fibre in the muscle spindle of the frog. Phil Trans Royal Soc Lond Ser B, Biol Sci. 1961;243:221-40.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

