Right median nerve electrical stimulation for acute traumatic coma (the Asia Coma Electrical Stimulation trial): study protocol for a randomised controlled trial
- PMID: 28693604
- PMCID: PMC5504835
- DOI: 10.1186/s13063-017-2045-x
Right median nerve electrical stimulation for acute traumatic coma (the Asia Coma Electrical Stimulation trial): study protocol for a randomised controlled trial
Abstract
Background: Traumatic brain injury (TBI) has become the most common cause of death and disability in persons between 15 and 30 years of age, and about 10-15% of patients affected by TBI will end up in a coma. Coma caused by TBI presents a significant challenge to neuroscientists. Right median nerve electrical stimulation has been reported as a simple, inexpensive, non-invasive technique to speed recovery and improve outcomes for traumatic comatose patients.
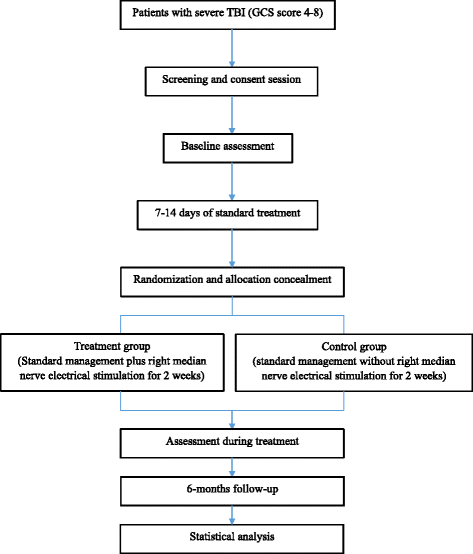
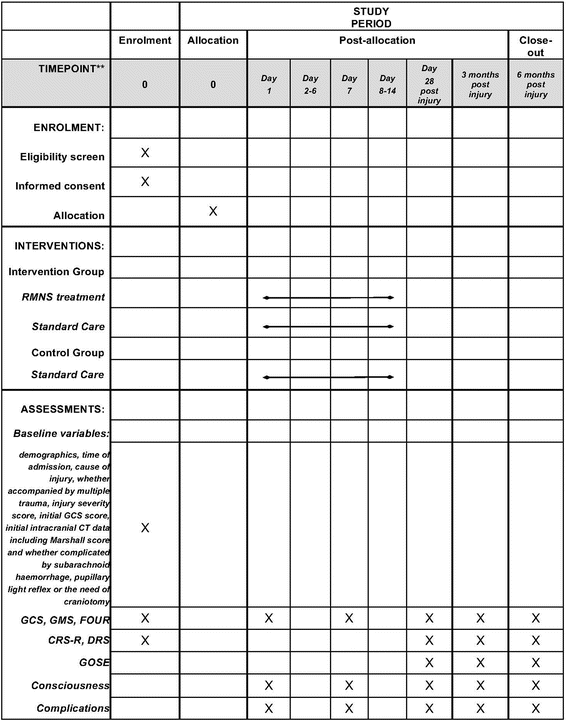
Methods/design: This multicentre, prospective, randomised (1:1) controlled trial aims to demonstrate the efficacy and safety of electrical right median nerve stimulation (RMNS) in both accelerating emergence from coma and promoting long-term outcomes. This trial aims to enrol 380 TBI comatose patients to partake in either an electrical stimulation group or a non-stimulation group. Patients assigned to the stimulation group will receive RMNS in addition to standard treatment at an amplitude of 15-20 mA with a pulse width of 300 μs at 40 Hz ON for 20 s and OFF for 40 s. The electrical treatment will last for 8 h per day for 2 weeks. The primary endpoint will be the percentage of patients regaining consciousness 6 months after injury. The secondary endpoints will be Extended Glasgow Outcome Scale, Coma Recovery Scale-Revised and Disability Rating Scale scores at 28 days, 3 months and 6 months after injury; Glasgow Coma Scale, Glasgow Coma Scale Motor Part and Full Outline of Unresponsiveness scale scores on day 1 and day 7 after enrolment and 28 days, 3 months and 6 months after injury; duration of unconsciousness and mechanical ventilation; length of intensive care unit and hospital stays; and incidence of adverse events.
Discussion: Right median nerve electrical stimulation has been used as a safe, inexpensive, non-invasive therapy for neuroresuscitation of coma patients for more than two decades, yet no trial has robustly proven the efficacy and safety of this treatment. The Asia Coma Electrical Stimulation (ACES) trial has the following novel features compared with other major RMNS trials: (1) the ACES trial is an Asian multicentre randomised controlled trial; (2) RMNS therapy starts at an early stage 7-14 days after the injury; and (3) various assessment scales are used to evaluate the condition of patients. We hope the ACES trial will lead to optimal use of right median nerve electrical treatment.
Trial registration: ClinicalTrials.gov, NCT02645578 . Registered on 23 December 2015.
Keywords: Comatose patient; Right median nerve electrical stimulation; Traumatic brain injury.
Figures
Similar articles
-
Acute traumatic coma awakening by right median nerve electrical stimulation: a randomised controlled trial.Intensive Care Med. 2023 Jun;49(6):633-644. doi: 10.1007/s00134-023-07072-1. Epub 2023 May 13. Intensive Care Med. 2023. PMID: 37178149 Free PMC article. Clinical Trial.
-
Right Median Nerve Electrical Stimulation for Acute Traumatic Coma Patients.J Neurotrauma. 2015 Oct 15;32(20):1584-9. doi: 10.1089/neu.2014.3768. Epub 2015 May 7. J Neurotrauma. 2015. PMID: 25664378
-
Efficacy and safety of electro-acupuncture treatment in improving the consciousness of patients with traumatic brain injury: study protocol for a randomized controlled trial.Trials. 2018 May 29;19(1):296. doi: 10.1186/s13063-018-2687-3. Trials. 2018. PMID: 29843761 Free PMC article.
-
Acute Traumatic Coma Awakening Induced by Median Nerve Electrical Stimulation: A Systematic Review and Meta-Analysis.Neurocrit Care. 2025 Jun;42(3):817-828. doi: 10.1007/s12028-024-02141-9. Epub 2024 Oct 24. Neurocrit Care. 2025. PMID: 39448428
-
[Research progress on median nerve electrical stimulation for awakening comatose patients with brain injury].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2024 Sep;36(9):997-1000. doi: 10.3760/cma.j.cn121430-20240617-00513. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2024. PMID: 39380525 Review. Chinese.
Cited by
-
Unlocking consciousness through right median nerve stimulation. Has a potential cure arrived at our doorstep?Intensive Care Med. 2023 Jun;49(6):659-661. doi: 10.1007/s00134-023-07097-6. Epub 2023 May 21. Intensive Care Med. 2023. PMID: 37210686 Free PMC article. No abstract available.
-
Safety of median nerve electrical stimulation in disorders of consciousness: A systematic review and meta-analysis of randomized controlled trials.PLoS One. 2025 Jul 31;20(7):e0324046. doi: 10.1371/journal.pone.0324046. eCollection 2025. PLoS One. 2025. PMID: 40743130 Free PMC article. Review.
-
Acute traumatic coma awakening by right median nerve electrical stimulation: a randomised controlled trial.Intensive Care Med. 2023 Jun;49(6):633-644. doi: 10.1007/s00134-023-07072-1. Epub 2023 May 13. Intensive Care Med. 2023. PMID: 37178149 Free PMC article. Clinical Trial.
-
An update on noninvasive neuromodulation in the treatment of patients with prolonged disorders of consciousness.CNS Neurosci Ther. 2024 May;30(5):e14757. doi: 10.1111/cns.14757. CNS Neurosci Ther. 2024. PMID: 38747078 Free PMC article. Review.
-
Emerging Treatments for Disorders of Consciousness in Paediatric Age.Brain Sci. 2022 Jan 31;12(2):198. doi: 10.3390/brainsci12020198. Brain Sci. 2022. PMID: 35203961 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

