Sialic acid linkage-specific permethylation for improved profiling of protein glycosylation by MALDI-TOF MS
- PMID: 28693729
- PMCID: PMC5953179
- DOI: 10.1016/j.aca.2017.05.029
Sialic acid linkage-specific permethylation for improved profiling of protein glycosylation by MALDI-TOF MS
Abstract
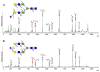
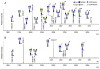
Protein glycosylation mediates a wide range of cellular processes, affecting development and disease in mammals. Deciphering the "glycocodes" requires rapid, sensitive and in-depth characterization of diverse glycan structures derived from biological samples. In this study, we described a two-step derivatization strategy termed linkage-specific sialic acid permethylation (SSAP) consisting of dimethylamination and permethylation for the improved profiling of glycosylation by matrix-assisted laser desorption/ionization (MALDI) time-of-fight (TOF) mass spectrometry (MS). High linkage-specificity (∼99%) of SSAP to both the two most common forms of sialic acid, N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc), permitted direct discrimination of α2,3- and α2,6-linked sialic acids in MALDI-TOF MS. The enhanced intensity (>10-fold) and increased detection limit (>10-fold) of derivatized glycans were valued for sensitive glycomics. Moreover, the good compatibility and reaction efficiency of the two steps of SSAP allowed rapid sample preparation (<2 h), benefiting robust analysis of glycans in a high-throughput manner. The SSAP strategy was further applied to investigate the protein glycosylation of human serum associated with rheumatoid arthritis (RA). It was demonstrated that the relative abundances of individual glycans were different in RA negative and RA positive samples, and meanwhile the RA patient/control ratios of both α2,3- and α2,6-sialylated glycans tended to elevate accompanied with the increase of sialylation. Those findings of the glycosylation changes occurred in human serum protein may contribute to the diagnosis of RA. Herein, SSAP derivatization combined with MALDI-TOF MS exhibits unique advantages for glycomic analysis and shows potential in glycosylation profiling of therapeutic proteins and clinical glycan biomarker discovery.
Keywords: Glycans; Glycosylation; MALDI-TOF MS; Rheumatoid arthritis; Sialic acid linkage-specific permethylation.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




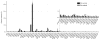

References
-
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. - PubMed
-
- Varki A, Freeze HH. Glycans in acquired human diseases. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. Cold Spring Harbor; NY: 2009. - PubMed
-
- Dube DH, Bertozzi CR. Glycans in cancer and inflammation–potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. - PubMed
-
- Jiang K, Aloor A, Qu J, Xiao C, Wu Z, Ma C, Zhang L, Wang PG. Rapid and sensitive MALDI MS analysis of oligosaccharides by using 2-hydrazinopyrimidine as a derivative reagent and co-matrix. Anal Bioanal Chem. 2017;409:421–429. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

